Heart Failure and Arrhythmias: Circadian and Epigenetic Interplay in Myocardial Electrophysiology
- PMID: 40141370
- PMCID: PMC11943068
- DOI: 10.3390/ijms26062728
Heart Failure and Arrhythmias: Circadian and Epigenetic Interplay in Myocardial Electrophysiology
Abstract
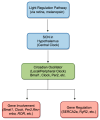
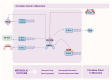
Emerging evidence underscores the impact of circadian rhythms on cardiovascular processes, particularly in conditions such as hypertension, myocardial infarction, and heart failure, where circadian rhythm disruptions are linked to disease progression and adverse clinical outcomes. Circadian clock proteins are intricately linked to myocardial electrophysiological remodeling and epigenetic pathways associated with arrhythmias in heart failure. In the context of heart failure, circadian clock dysregulation leads to electrophysiological remodeling in the cardiomyocytes, which can precipitate life-threatening arrhythmias such as ventricular tachycardia (VT) and ventricular fibrillation (VF). This dysregulation may be influenced by environmental factors, such as diet and exercise, as well as genetic factors. Moreover, epigenetic modifications in heart failure have been implicated in the regulation of genes involved in cardiac hypertrophy, fibrosis, and inflammation. The interplay between circadian clock proteins, myocardial electrophysiological remodeling, and epigenetic pathways in heart failure-related arrhythmias is complex and multifaceted. Further research is needed to elucidate how these processes interact and contribute to the development of arrhythmias in heart failure patients. This review aims to explore the connections between circadian rhythms, myocardial electrophysiology, and arrhythmias related to heart failure, with the goal of identifying potential therapeutic targets and interventions that may counteract the adverse effects of circadian disruptions on cardiovascular health.
Keywords: circadian clock; circadian proteins; epigenetic regulations; heart failure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Zeppenfeld K., Tfelt-Hansen J., de Riva M., Winkel B.G., Behr E.R., Blom N.A., Charron P., Corrado D., Dagres N., de Chillou C., et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022;43:3997–4126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

