Identification of a Novel Antagonist of BRS-3 from Natural Products and Its Protective Effects Against H2O2-Induced Cardiomyocyte Injury
- PMID: 40141387
- PMCID: PMC11943355
- DOI: 10.3390/ijms26062745
Identification of a Novel Antagonist of BRS-3 from Natural Products and Its Protective Effects Against H2O2-Induced Cardiomyocyte Injury
Abstract
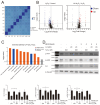
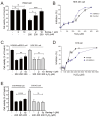
The identification of exogenous ligands from natural products is an alternative strategy to explore the unrevealed physiological functions of orphan G-protein-coupled receptors (GPCRs). In this study, we have successfully identified and pharmacologically characterized licoisoflavone A (LIA) as a novel selective antagonist of BRS-3, an orphan GPCR. Functional studies showed that pretreatment with LIA ameliorated hydrogen peroxide (H2O2)-induced cardiomyocyte injury. Furthermore, LIA pretreatment significantly restored the activities of malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT), as well as lactate dehydrogenase (LDH) levels, in H9c2 cells following H2O2 exposure. The protective effect of LIA was also evident in primary cardiomyocytes from rats and mice against H2O2-induced cell injury but was absent in primary cardiomyocytes derived from bombesin receptor subtype-3 knockout (Brs3-/y) mice, strongly confirming the mechanism of LIA's action through BRS-3 antagonism. Proteomics studies further revealed that LIA exerted its protective effects via activating the integrin/ILK/AKT and ERK/MAPK signaling pathways. Complementary findings from Bantag-1, a well-recognized antagonist of BRS-3, in human embryonic kidney 293 mBRS-3 (HEK293-mBRS-3) stable cells and B16 cell lines, which demonstrated resistance to H2O2-induced damage, further supported the pivotal role of BRS-3 in oxidative stress-induced cell injury. Our study contributes to expanding our understanding of the potential pharmacological functions of BRS-3, unveiling previously unknown pharmacological functionality of this orphan receptor.
Keywords: BRS-3; antagonist; cardiomyocyte; licoisoflavone A; orphan GPCR.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Protective effect of tanshinone IIA on H2O2-induced oxidative stress injury in rat cardiomyocytes by activating Nrf2 pathway.J Recept Signal Transduct Res. 2020 Jun;40(3):264-272. doi: 10.1080/10799893.2020.1731535. Epub 2020 Feb 26. J Recept Signal Transduct Res. 2020. PMID: 32100629
-
Efficacy of water fraction from Dioscorea cirrhosa on oxidative stress and apoptosis in H9c2 cardiomyocytes induced by H2O2.J Tradit Chin Med. 2021 Feb;41(1):51-58. doi: 10.19852/j.cnki.jtcm.2021.01.007. J Tradit Chin Med. 2021. PMID: 33522197
-
FGF-2 Transcriptionally Down-Regulates the Expression of BNIP3L via PI3K/Akt/FoxO3a Signaling and Inhibits Necrosis and Mitochondrial Dysfunction Induced by High Concentrations of Hydrogen Peroxide in H9c2 Cells.Cell Physiol Biochem. 2016;40(6):1678-1691. doi: 10.1159/000453217. Epub 2016 Dec 23. Cell Physiol Biochem. 2016. PMID: 28006775
-
Schisandra chinensis bee pollen's chemical profiles and protective effect against H2O2-induced apoptosis in H9c2 cardiomyocytes.BMC Complement Med Ther. 2020 Sep 10;20(1):274. doi: 10.1186/s12906-020-03069-1. BMC Complement Med Ther. 2020. PMID: 32912207 Free PMC article.
-
Cystatin C alleviates H2O2-induced H9c2 cell injury.Eur Rev Med Pharmacol Sci. 2020 Jun;24(11):6360-6370. doi: 10.26355/eurrev_202006_21534. Eur Rev Med Pharmacol Sci. 2020. PMID: 32572933
Cited by
-
An Agonist of Zinc-Sensing G-Protein Coupled Receptor 39 Accelerates Skin Wound Healing and Protects Against UVB-Induced Keratinocyte Damage.J Exp Pharmacol. 2025 Aug 13;17:571-585. doi: 10.2147/JEP.S531431. eCollection 2025. J Exp Pharmacol. 2025. PMID: 40827131 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

