Impact of the Human Leukocyte Antigen Complex on Idiopathic Pulmonary Fibrosis Development and Progression in the Sardinian Population
- PMID: 40141400
- PMCID: PMC11942992
- DOI: 10.3390/ijms26062760
Impact of the Human Leukocyte Antigen Complex on Idiopathic Pulmonary Fibrosis Development and Progression in the Sardinian Population
Abstract
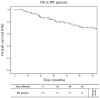
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease characterized by the disruption of the alveolar and interstitial architecture due to extracellular matrix deposition. Emerging evidence suggests that genetic susceptibility plays a crucial role in IPF development. This study explores the role of human leukocyte antigen (HLA) alleles and haplotypes in IPF susceptibility and progression within the genetically distinct Sardinian population. Genotypic data were analyzed for associations with disease onset and progression, focusing on allele and haplotype frequencies in patients exhibiting slow (S) or rapid (R) progression. While no significant differences in HLA allele frequencies were observed between IPF patients and controls, the HLA-DRB1*04:05 allele and the extended haplotype (HLA-A*30:02, B*18:01, C*05:01, DQA1*05:01, DQB1*02:01, DRB1*03:01) were associated with a slower disease progression and improved survival (log-rank = 0.032 and 0.01, respectively). At 36 months, carriers of these variants demonstrated significantly better pulmonary function, measured with single-breath carbon monoxide diffusing capacity (DLCO%p) (p = 0.005 and 0.02, respectively). Multivariate analysis confirmed these findings as being independent of confounding factors. These results highlight the impact of HLA alleles and haplotypes on IPF outcomes and underscore the potential of the Sardinian genetic landscape to illuminate immunological mechanisms, paving the way for predictive biomarkers and personalized therapies.
Keywords: HLA; ILDs; IPF; Sardinia; genetics; idiopathic pulmonary fibrosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

