A Benzodiazepine-Derived Molecule That Interferes with the Bio-Mechanical Properties of Glioblastoma-Astrocytoma Cells Altering Their Proliferation and Migration
- PMID: 40141408
- PMCID: PMC11943291
- DOI: 10.3390/ijms26062767
A Benzodiazepine-Derived Molecule That Interferes with the Bio-Mechanical Properties of Glioblastoma-Astrocytoma Cells Altering Their Proliferation and Migration
Abstract
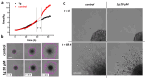
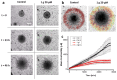
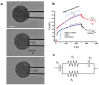
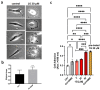
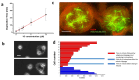
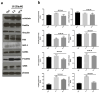
Glioblastoma multiforme (grade IV glioma) is characterized by a high invasive potential, making surgical intervention extremely challenging and patient survival very limited. Current pharmacological approaches show, at best, slight improvements in the therapy against this type of tumor. Microtubules are often the target of antitumoral drugs, and specific drugs affecting their dynamics by acting on microtubule-associated proteins (MAPs) without producing their depolymerization could affect both glioma cell migration/invasion and cell proliferation. Here, we analyzed on a cellular model of glioblastoma multiforme, the effect of a molecule (1-(4-amino-3,5-dimethylphenyl)-3,5-dihydro-7,8-ethylenedioxy-4h2,3-benzodiazepin-4-one, hereafter named 1g) which was shown to act as a cytostatic drug in other cell types by affecting microtubule dynamics. We found that the molecule acts also as a migration suppressor by inducing a loss of cell polarity. We characterized the mechanics of U87MG cell aggregates exposed to 1g by different biophysical techniques. We considered both 3D aggregates and 2D cell cultures, testing substrates of different stiffness. We established that this molecule produces a decrease of cell spheroid contractility and it impairs 3D cell invasion. At the same time, in the case of isolated cells, 1g selectively produces an almost instantaneous loss of cell polarity blocking migration and it also produces a disorganization of the mitotic spindle when cells reach mitosis, leading to frequent mitotic slippage events followed by cell death. We can state that the studied molecule produces similar effects to other molecules that are known to affect the dynamics of microtubules, but probably indirectly via microtubule-associated proteins (MAPs) and following different biochemical pathways. Consistently, we report evidence that, regarding its effect on cell morphology, this molecule shows a specificity for some cell types such as glioma cells. Interestingly, being a molecule derived from a benzodiazepine, the 1g chemical structure could allow this molecule to easily cross the blood-brain barrier. Thanks to its chemical/physical properties, the studied molecule could be a promising new drug for the specific treatment of GBM.
Keywords: GBM; anticancer drug; biomechanics; invasion; microtubule; spheroid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



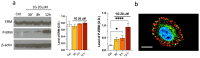
References
-
- Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J., Wolinsky Y., Kruchko C., Barnholtz-Sloan J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology. 2014;16:iv1–iv63. doi: 10.1093/neuonc/nou223. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

