The Crosstalk Between NETs and the Complement Cascade: An Overview in Nephrological Autoimmune Disease
- PMID: 40141431
- PMCID: PMC11943363
- DOI: 10.3390/ijms26062789
The Crosstalk Between NETs and the Complement Cascade: An Overview in Nephrological Autoimmune Disease
Abstract
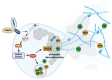
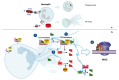
The complement cascade and Neutrophil Extracellular Traps (NETs) represent fundamental tools in protecting the host from foreign pathogens. Complement components and relative fragments, classically assigned to the innate immunity, represent a key link with the humoral immune response. NETs are a crucial component of the innate immune response, consisting of chromatin release from activated neutrophils. These web-like structures facilitate pathogen entrapment and elimination through proteolytic degradation and antimicrobial effectors. Previous findings suggested complement components and NETs have a significant role in the pathogenesis of several diseases characterized by inflammation, such as autoimmune and infectious diseases. However, the crosstalk between NETs and the complement cascade has only recently been investigated, and several aspects still need to be fully clarified. Recent evidence seems to suggest a bidirectional link between the complement cascade and NETosis. We here present the interaction between complement components and NETs in specific autoimmune diseases that mostly affect the kidney, such as systemic lupus erythematosus, Antineutrophilic Cytoplasmic Antibody (ANCA)-associated vasculitis and antiphospholipid syndrome. The mechanisms reported here may represent specific targets for the development of possible therapeutic strategies.
Keywords: ANCA vasculitis; Neutrophil Extracellular Traps; Systemic Lupus Erythematosus; antiphospholipid syndrome; autoimmunity; avacopan; complement cascade; eculizumab.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

