Harnessing Plasma Biomarkers to Predict Immunotherapy Outcomes in Hepatocellular Carcinoma: The Role of cfDNA, ctDNA, and Cytokines
- PMID: 40141436
- PMCID: PMC11942713
- DOI: 10.3390/ijms26062794
Harnessing Plasma Biomarkers to Predict Immunotherapy Outcomes in Hepatocellular Carcinoma: The Role of cfDNA, ctDNA, and Cytokines
Abstract
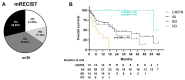
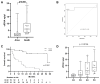
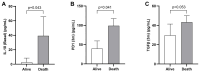
Immunotherapy has improved survival in patients with advanced hepatocellular carcinoma (HCC); yet, objective radiological responses occur in only about 20% of cases, suggesting variable benefits. This study aimed to identify serologic markers predictive of response to immune checkpoint inhibitors (ICIs). A cohort of 38 advanced HCC patients receiving immunotherapy was prospectively analyzed. Levels of cell-free DNA (cfDNA), circulating tumor DNA (ctDNA), and cytokines were measured pre-treatment and three months post-treatment initiation. Genomic profiling of ctDNA was also conducted. Baseline levels of cfDNA and ctDNA effectively discriminated HCC patients based on their radiological response to ICIs. Additionally, individuals with pathologic mutations in the CDKN2A gene exhibited significantly reduced survival. Patients with progressive disease (PD) as their best radiological response had significantly fewer copy number variations (CNVs) than those with a radiological response. Furthermore, levels of IL10, PD1, and TGFβ assessed after three months of treatment showed significant variations correlating with survival status. In conclusion, the analysis of cfDNA, ctDNA, and cytokines may improve treatment selection for HCC patients by predicting their expected response to immunotherapies.
Keywords: biomarkers; cfDNA; ctDNA; hepatocellular carcinoma; immunotherapy; liquid biopsy.
Conflict of interest statement
B.M. received consultancy fees from Bayer–Shering Pharma, Roche, Astra Zeneca and Eisai–Merck, lectures/speaker fees from Eisai–MSD, Astra Zeneca, Bayer–Shering, and Roche, and a research grant from Laboratorios Viñas S.L. The remaining authors have no conflicts of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- IARC. [(accessed on 18 October 2024)]. Available online: http://globocan.iarc.fr/pages/fact_sheets_population.aspx.
-
- Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
-
- El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.Y., Choo S.P., Trojan J., Welling T.H., et al. Nivolumab in Patients with Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. - DOI - PMC - PubMed
-
- Finn R.S., Ryoo B.Y., Merle P., Kudo M., Bouattour M., Lim H.Y., Breder V., Edeline J., Chao Y., Ogasawara S., et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. - DOI - PubMed
-
- Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., Kudo M., Harding J.J., Merle P., Rosmorduc O., et al. Nivolumab versus Sorafenib in Advanced Hepatocellular Carcinoma (CheckMate 459): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

