Emerging Ferroptosis Involvement in Amyotrophic Lateral Sclerosis Pathogenesis: Neuroprotective Activity of Polyphenols
- PMID: 40141987
- PMCID: PMC11944684
- DOI: 10.3390/molecules30061211
Emerging Ferroptosis Involvement in Amyotrophic Lateral Sclerosis Pathogenesis: Neuroprotective Activity of Polyphenols
Abstract
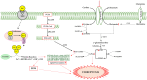
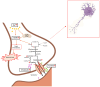
Neurodegenerative diseases are a group of diseases that share common features, such as the generation of misfolded protein deposits and increased oxidative stress. Among them, amyotrophic lateral sclerosis (ALS), whose pathogenesis is still not entirely clear, is a complex neurodegenerative disease linked both to gene mutations affecting different proteins, such as superoxide dismutase 1, Tar DNA binding protein 43, Chromosome 9 open frame 72, and Fused in Sarcoma, and to altered iron homeostasis, mitochondrial dysfunction, oxidative stress, and impaired glutamate metabolism. The purpose of this review is to highlight the molecular targets common to ALS and ferroptosis. Indeed, many pathways implicated in the disease are hallmarks of ferroptosis, a recently discovered type of iron-dependent programmed cell death characterized by increased reactive oxygen species (ROS) and lipid peroxidation. Iron accumulation results in mitochondrial dysfunction and increased levels of ROS, lipid peroxidation, and ferroptosis triggers; in addition, the inhibition of the Xc- system results in reduced cystine levels and glutamate accumulation, leading to excitotoxicity and the inhibition of GPx4 synthesis. These results highlight the potential involvement of ferroptosis in ALS, providing new molecular and biochemical targets that could be exploited in the treatment of the disease using polyphenols.
Keywords: cell death; glutathione peroxidase 4; natural compounds; neurodegenerative diseases; oxidative stress; system Xc−.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Manjaly Z.R., Scott K.M., Abhinav K., Wijesekera L., Ganesalingam J., Goldstein L.H., Janssen A., Dougherty A., Willey E., Stanton B.R., et al. The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotroph. Lateral Scler. 2010;11:439–442. doi: 10.3109/17482961003610853. - DOI - PMC - PubMed
-
- Marin B., Hamidou B., Couratier P., Nicol M., Delzor A., Raymondeau M., Druet-Cabanac M., Lautrette G., Boumediene F., Preux P.M., et al. Population-based epidemiology of amyotrophic lateral sclerosis (ALS) in an ageing Europe—The French register of ALS in Limousin (FRALim register) Eur. J. Neurol. 2014;21:1292-e79. doi: 10.1111/ene.12474. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

