Polythiophene/Ti3C2TX MXene Composites for Effective Removal of Diverse Organic Dyes via Complementary Activity of Adsorption and Photodegradation
- PMID: 40142169
- PMCID: PMC11944630
- DOI: 10.3390/molecules30061393
Polythiophene/Ti3C2TX MXene Composites for Effective Removal of Diverse Organic Dyes via Complementary Activity of Adsorption and Photodegradation
Abstract
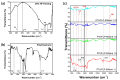
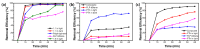
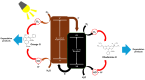
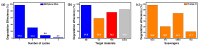
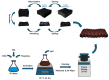
This study presents an effective method to remove organic dyes from wastewater, using a composite of few-layered porous (FLP) Ti3C2Tx MXene and polythiophene (PTh) nanospheres. The FLP MXene, which was pre-synthesized by a series of intercalation, heat-induced TiO2 formation, and its selective etching, was combined with PTh nanospheres via a simple solution method. The composite effectively removed various organic dyes, but its efficiency was altered depending on the type of dye. Particularly, the removal efficiency of methylene blue reached 91.3% and 97.8% after irradiation for 10 min and 1 h, respectively. The high dye removal efficiency was attributed to the large surface area (32.01 m2/g) of the composite, strong electrostatic interaction between the composite and dye molecules, and active photodegradation process. The strong electrostatic interaction and large surface area could facilitate the adsorption of dye molecules, while photocatalytic activity further enhance dye removal under light. These results are indicative that the PTh/FLP MXene composite may be a promising material for environmental remediation through synergistic processes of adsorption and photocatalysis.
Keywords: Ti3C2TX MXene; adsorption; dye removal; photocatalyst; polythiophene nanospheres.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Zia J., Fatima F., Riaz U. A comprehensive review on the photocatalytic activity of polythiophene-based nanocomposites against degradation of organic pollutants. Catal. Sci. Technol. 2021;11:6630–6648.
-
- Dutta K., Rana D. Polythiophenes: An emerging class of promising water purifying materials. Eur. Polym. J. 2019;119:370–385.
-
- Mashtalir O., Cook K.M., Mochalin V.N., Crowe M., Barsoum M.W., Gogotsi Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A. 2014;2:14334–14338. doi: 10.1039/C4TA02638A. - DOI
-
- You Z., Liao Y., Li X., Fan J., Xiang Q. State-of-the-art recent progress in MXene-based photocatalysts: A comprehensive review. Nanoscale. 2021;13:9463–9504. - PubMed
-
- Li X., Bai Y., Shi X., Su N., Nie G., Zhang R., Nie H., Ye L. Applications of MXene (Ti3C2TX) in photocatalysis: A review. Mater. Adv. 2021;2:1570–1594.
Grants and funding
LinkOut - more resources
Full Text Sources

