Itaconate: A Nexus Metabolite Fueling Leishmania Survival Through Lipid Metabolism Modulation
- PMID: 40142422
- PMCID: PMC11944847
- DOI: 10.3390/microorganisms13030531
Itaconate: A Nexus Metabolite Fueling Leishmania Survival Through Lipid Metabolism Modulation
Abstract
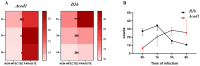
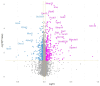
Leishmaniasis, caused by the Leishmania parasite, is a neglected public health issue. Leishmania mainly infects macrophages, where metabolic reprogramming shapes their plasticity (M1/M2), affecting the host's resistance or susceptibility to infection. The development of this infection is influenced by immune responses, with an excessive anti-inflammatory reaction linked to negative outcomes through the modulation of various mediators. Itaconate, produced by the Acod1 gene, is recognized for its anti-inflammatory effects, but its function in leishmaniasis is not well understood. This study aimed to investigate the potential role of itaconate in leishmaniasis. Using transcriptomic data from L. major-infected BMDMs, we assessed the expression dynamics of Il1b and Acod1 and performed pathway enrichment analysis to determine the profile of genes co-expressed with Acod1. Early Acod1 upregulation followed by later Il1b downregulation was noted, indicating a shift towards an anti-inflammatory response. Among the genes co-expressed with Acod1, Ldlr, Hadh, and Src are closely associated with lipid metabolism and the polarization of macrophages towards the M2 phenotype, thereby creating a favorable environment for the survival of Leishmania. Overall, these findings suggest that Acod1 and its co-expressed genes may affect the outcome of Leishmania infection by modulating host metabolism. Accordingly, targeting itaconate-associated pathways could provide a novel therapeutic strategy for leishmaniasis.
Keywords: Acod1; Hadh; IL1b; Ldlr; Leishmania; M1/M2 macrophages; Src; itaconate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Krawczyk C.M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R.J., Cross J.R., Jung E., Thompson C.B., Jones R.G., et al. Toll-like Receptor–Induced Changes in Glycolytic Metabolism Regulate Dendritic Cell Activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

