Engineering Useful Microbial Species for Pharmaceutical Applications
- PMID: 40142492
- PMCID: PMC11944651
- DOI: 10.3390/microorganisms13030599
Engineering Useful Microbial Species for Pharmaceutical Applications
Abstract
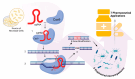
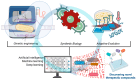
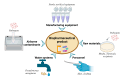
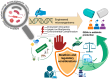
Microbial engineering has made a significant breakthrough in pharmaceutical biotechnology, greatly expanding the production of biologically active compounds, therapeutic proteins, and novel drug candidates. Recent advancements in genetic engineering, synthetic biology, and adaptive evolution have contributed to the optimization of microbial strains for pharmaceutical applications, playing a crucial role in enhancing their productivity and stability. The CRISPR-Cas system is widely utilized as a precise genome modification tool, enabling the enhancement of metabolite biosynthesis and the activation of synthetic biological pathways. Additionally, synthetic biology approaches allow for the targeted design of microorganisms with improved metabolic efficiency and therapeutic potential, thereby accelerating the development of new pharmaceutical products. The integration of artificial intelligence (AI) and machine learning (ML) plays a vital role in further advancing microbial engineering by predicting metabolic network interactions, optimizing bioprocesses, and accelerating the drug discovery process. However, challenges such as the efficient optimization of metabolic pathways, ensuring sustainable industrial-scale production, and meeting international regulatory requirements remain critical barriers in the field. Furthermore, to mitigate potential risks, it is essential to develop stringent biocontainment strategies and implement appropriate regulatory oversight. This review comprehensively examines recent innovations in microbial engineering, analyzing key technological advancements, regulatory challenges, and future development perspectives.
Keywords: artificial intelligence; biopharmaceuticals; machine learning; microbial engineering; synthetic biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Unlocking the potential of biosurfactants: Innovations in metabolic and genetic engineering for sustainable industrial and environmental solutions.Biotechnol Notes. 2024 Jul 25;5:111-119. doi: 10.1016/j.biotno.2024.07.001. eCollection 2024. Biotechnol Notes. 2024. PMID: 39416688 Free PMC article. Review.
-
Microbial Transcription Factor-Based Biosensors: Innovations from Design to Applications in Synthetic Biology.Biosensors (Basel). 2025 Mar 31;15(4):221. doi: 10.3390/bios15040221. Biosensors (Basel). 2025. PMID: 40277535 Free PMC article. Review.
-
Revolutionizing Molecular Design for Innovative Therapeutic Applications through Artificial Intelligence.Molecules. 2024 Sep 29;29(19):4626. doi: 10.3390/molecules29194626. Molecules. 2024. PMID: 39407556 Free PMC article. Review.
-
Advancing microbial production through artificial intelligence-aided biology.Biotechnol Adv. 2024 Sep;74:108399. doi: 10.1016/j.biotechadv.2024.108399. Epub 2024 Jun 24. Biotechnol Adv. 2024. PMID: 38925317 Review.
-
Strategies in engineering sustainable biochemical synthesis through microbial systems.Curr Opin Chem Biol. 2024 Aug;81:102493. doi: 10.1016/j.cbpa.2024.102493. Epub 2024 Jul 5. Curr Opin Chem Biol. 2024. PMID: 38971129 Review.
Cited by
-
Key Insights into Gut Alterations in Metabolic Syndrome.J Clin Med. 2025 Apr 14;14(8):2678. doi: 10.3390/jcm14082678. J Clin Med. 2025. PMID: 40283508 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

