Catheter Ablation of Atrial Fibrillation: Technique and Future Perspectives
- PMID: 40142600
- PMCID: PMC11943125
- DOI: 10.3390/jcm14061788
Catheter Ablation of Atrial Fibrillation: Technique and Future Perspectives
Abstract
Atrial fibrillation is the most common sustained cardiac arrhythmia with a significant impact on quality of life in terms of symptoms and reduction of functional status. Also, it is associated with an increased risk of mortality, stroke, and peripheral embolism. Catheter ablation for atrial fibrillation has become a well-established treatment, improving arrhythmia outcomes without increasing the risk of serious adverse events compared to antiarrhythmic drug therapy. The field has undergone significant advancements in recent years, yet pulmonary vein isolation continues to be the cornerstone of any atrial fibrillation ablation procedure. The purpose of this review is to provide an overview of the current techniques, emerging technologies, and future directions.
Keywords: atrial fibrillation; catheter ablation; posterior wall isolation; pulmonary vein isolation; pulsed field; vein of Marshall.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

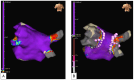



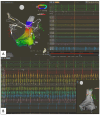


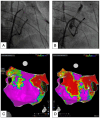

References
-
- Holmes D.N., Piccini J.P., Allen L.A., Fonarow G.C., Gersh B.J., Kowey P.R., O’Brien E.C., Reiffel J.A., Naccarelli G.V., Ezekowitz M.D., et al. Defining Clinically Important Difference in the Atrial Fibrillation Effect on Quality-of-Life Score. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005358. doi: 10.1161/CIRCOUTCOMES.118.005358. - DOI - PubMed
-
- Bassand J.-P., Accetta G., Camm A.J., Cools F., Fitzmaurice D.A., Fox K.A.A., Goldhaber S.Z., Goto S., Haas S., Hacke W., et al. Two-Year Outcomes of Patients with Newly Diagnosed Atrial Fibrillation: Results from GARFIELD-AF. Eur. Heart J. 2016;37:2882–2889. doi: 10.1093/eurheartj/ehw233. - DOI - PMC - PubMed
-
- Wilber D.J., Pappone C., Neuzil P., De Paola A., Marchlinski F., Natale A., Macle L., Daoud E.G., Calkins H., Hall B., et al. Comparison of Antiarrhythmic Drug Therapy and Radiofrequency Catheter Ablation in Patients with Paroxysmal Atrial Fibrillation. JAMA. 2010;303:333. doi: 10.1001/jama.2009.2029. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

