Clinical Utilization and Performance of Bempedoic Acid in an Italian Real-World Setting: Insight from Campania Region
- PMID: 40142647
- PMCID: PMC11943254
- DOI: 10.3390/jcm14061839
Clinical Utilization and Performance of Bempedoic Acid in an Italian Real-World Setting: Insight from Campania Region
Abstract
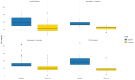
Background/Objectives: Bempedoic acid (BA) is a novel lipid-lowering agent that reduces low-density lipoprotein cholesterol (LDL-c) and cardiovascular events. Limited real-world data on its effectiveness and safety are available. This study aimed to evaluate the utilization and clinical performance of BA in routine clinical practice. Moreover, an explorative pharmacoeconomic analysis was performed. Methods: We prospectively enrolled consecutive patients with dyslipidemia who started 180 mg BA, alone or with 10 mg ezetimibe, across five outpatient clinics in Campania Region, Italy from September to December 2023. Clinical and laboratory assessments, including lipid profile, hepatic function, and creatine phosphokinase levels, were performed at baseline and at least after one month follow-up. Side effects were recorded. Results: 111 patients (age 65 ± 9 years, 61% male) were included. At BA initiation, 70.3% were on maximally tolerated statin dosage and ezetimibe, 16.2% on ezetimibe alone, and 13.5% on PCSK9 inhibitors due to statin intolerance. BA significantly reduced LDL-c serum levels (89.9 ± 33.0 vs. 56 ± 27.6 mg/dL; p < 0.0001), with 46% achieving therapeutic targets. LDL-c decreased by 28% in patients on intensive statins/ezetimibe and by 45% in statin-intolerant patients, with reduced healthcare costs. Side effects were infrequent (10%) and reversible. Adherence was 99%, and persistence 90%. Conclusions: In our clinical pratice, BA was primarily used in high-risk patients with dyslipidemia who failed to reach LDL-c therapeutic target with statins/ezetimibe, and to a lesser extent, in statin-intolerant individuals. BA treatment enabled 54% to reach LDL-c therapeutic target. BA was well tolerated, and showed high adherence and persistence, contributing to cost savings.
Keywords: adherence; bempedoic acid; cardiovascular risk; lipid-lowering therapy; low-density lipoprotein cholesterol (LDL-c); persistence; side effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Barrios V., Pintó X., Escobar C., Varona J.F., Gámez J.M. Real-World Attainment of Low-Density Lipoprotein Cholesterol Goals in Patients at High Risk of Cardiovascular Disease Treated with High-Intensity Statins: The TERESA Study. J. Clin. Med. 2023;12:3187. doi: 10.3390/jcm12093187. - DOI - PMC - PubMed
-
- American College of Cardiology ASCVD Patients Face Barriers Reaching Guideline-Recommended Cholesterol Goals. [(accessed on 20 February 2025)]. Available online: https://www.acc.org/about-acc/press-releases/2019/11/13/15/38/ascvd-pati....
-
- Goldberg A.C., Leiter L.A., Stroes E.S., Baum S.J., Hanselman J.C., Bloedon L.T., Duell P.B. Effect of bempedoic acid vs. placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: The CLEAR Wisdom randomized clinical trial. JAMA. 2019;322:1780–1788. doi: 10.1001/jama.2019.16585. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

