Severely Ill COVID-19 Patients May Exhibit Hypercoagulability Despite Escalated Anticoagulation
- PMID: 40142778
- PMCID: PMC11943368
- DOI: 10.3390/jcm14061966
Severely Ill COVID-19 Patients May Exhibit Hypercoagulability Despite Escalated Anticoagulation
Abstract
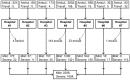
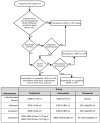
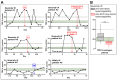
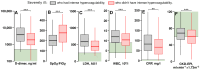
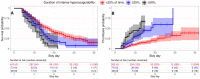
Introduction: Severely ill COVID-19 patients receiving prophylactic-dose anticoagulation exhibit high rates of thrombosis and mortality. The escalation of anticoagulation also does not reduce mortality and has an uncertain impact on thrombosis rates. The reasons why escalated doses fail to outperform prophylactic doses in reducing risks of thrombosis and death in severely ill COVID-19 patients remain unclear. We hypothesized that escalated anticoagulation would not effectively prevent hypercoagulability and, consequently, would not reduce the risk of thrombosis and death in some severely ill patients. Methods: We conducted a prospective multicenter study that enrolled 3860 COVID-19 patients, including 1654 severely ill. They received different doses of low-molecular-weight or unfractionated heparin, and their blood coagulation was monitored with activated partial thromboplastin time, D-dimer, and Thrombodynamics. A primary outcome was hypercoagulability detected by Thrombodynamics. Blood samples were collected at the trough level of anticoagulation. Results: We found that escalated anticoagulation did not prevent hypercoagulability in 28.3% of severely ill patients at the trough level of the pharmacological activity. Severely ill patients with such hypercoagulability had higher levels of inflammation markers and better creatinine clearance compared to severely ill patients without it. Hypercoagulability detected by Thrombodynamics was associated with a 1.68-fold higher hazard rate for death and a 3.19-fold higher hazard rate for thrombosis. Elevated D-dimer levels were also associated with higher hazard rates for thrombosis and death, while shortened APTTs were not. The simultaneous use of Thrombodynamics and D-dimer data enhanced the accuracy for predicting thrombotic events and fatal outcomes in severely ill patients. Conclusions: Thrombodynamics reliably detects hypercoagulability in COVID-19 patients and can be used in conjunction with D-dimer to assess the risk of thrombosis and death in severely ill patients. The pharmacological effect of LMWH at the trough level might be too low to prevent thrombosis in some severely ill patients with severe inflammation and better creatinine clearance, even if escalated doses are used.
Keywords: LMWH; Thrombodynamics; UFH; thromboprophylaxis.
Conflict of interest statement
The authors and COVITRO investigators have declared no competing financial interests or personal relationships that could have influenced the study.
Figures

References
-
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. - DOI - PMC - PubMed
-
- van den Berg J., Haslbauer J.D., Stalder A.K., Romanens A., Mertz K.D., Studt J.D., Siegemund M., Buser A., Holbro A., Tzankov A. Von Willebrand factor and the thrombophilia of severe COVID-19: In situ evidence from autopsies. Res. Pract. Thromb. Haemost. 2023;7:100182. doi: 10.1016/j.rpth.2023.100182. - DOI - PMC - PubMed
-
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

