Long-Term Clinical Remission on Benralizumab Treatment in Severe Eosinophilic Asthma: A Four-Year Real-Life Study
- PMID: 40142883
- PMCID: PMC11942882
- DOI: 10.3390/jcm14062075
Long-Term Clinical Remission on Benralizumab Treatment in Severe Eosinophilic Asthma: A Four-Year Real-Life Study
Abstract
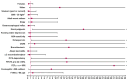
Background: The current availability of monoclonal antibodies against key mediators of type-2 (T2) inflammation has led to a redefinition of the ultimate objectives of severe asthma treatment to a more composite concept of disease remission. Objectives: The aim of this real-life study was to estimate the percentage of patients who achieved clinical remission over 4 years of treatment with benralizumab, and to identify baseline predictors for the achievement of such a composite outcome in the long term. Methods: Data from a 4-year follow-up of 23 patients who were prescribed benralizumab as an add-on therapy because of uncontrolled severe eosinophilic asthma were retrospectively analyzed and compared. Clinical remission was considered to be "complete" if oral corticosteroid (OCS) use was not required, there were no exacerbations, an asthma control test (ACT) score ≥ 20 was achieved and a pre-bronchodilation percent predicted a forced expiratory volume in 1 s (FEV1%) ≥ 80%. Clinical remission was considered to be "partial" if OCS use was not required, plus at least two of the other three aforementioned criteria. Results: The overall percentage of patients who achieved clinical remission was 86.9% after 12 months, and 91.3% after 24 and 48 months of treatment. The rate of complete remission over partial remission increased over time. After 12 months of treatment, 65% of patients fulfilled the criteria for complete remission and 35.0% for partial remission. After 48 months of treatment, 71.4% of patients were in a status of complete remission and 28.6% in a status of partial remission. A long-term composite outcome of complete clinical remission was more likely to be achieved by severe eosinophilic asthma patients with comorbid nasal polyposis, bronchiectasis and osteoporosis, and with OCS dependency, a predicted pre-bronchodilation FEV1% ≥ 80% and a predicted FEF25-75% < 65% at baseline. Conclusions: Our real-life experience suggests that treatment with benralizumab may allow the achievement and long-term maintenance of clinical remission in a high percentage of severe eosinophilic asthma patients, up to 4 years of follow-up.
Keywords: asthma; benralizumab; non-invasive biomarkers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- 2023 GINA Main Report—Global Initiative for Asthma—GINA. [(accessed on 12 March 2024)]. Available online: https://ginasthma.org/2023-gina-main-report/
-
- Lefebvre P., Duh M.S., Lafeuille M.H., Gozalo L., Desai U., Robitaille M.N., Albers F., Yancey S., Ortega H., Forshag M., et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J. Allergy Clin. Immunol. 2015;136:1488–1495. doi: 10.1016/j.jaci.2015.07.046. - DOI - PubMed
-
- The Global Asthma Report 2022. [(accessed on 12 March 2025)]. Available online: https://globalasthmareport.org/burden/burden.php.
LinkOut - more resources
Full Text Sources

