Effectiveness of Tralokinumab Across Atopic Dermatitis Phenotypes
- PMID: 40142885
- PMCID: PMC11942799
- DOI: 10.3390/jcm14062077
Effectiveness of Tralokinumab Across Atopic Dermatitis Phenotypes
Abstract
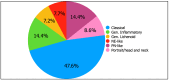
Background/Objectives: Tralokinumab, a fully human monoclonal antibody targeting IL-13, has shown efficacy and safety in clinical trials and real-life studies for atopic dermatitis (AD). However, data on its effectiveness across AD phenotypes are limited. Methods: A multicentric study evaluated tralokinumab's efficacy over 52 weeks in 416 severe AD patients. EASI (Eczema Area and Severity Index), P-NRS (Pruritus Numerical Rating Scale), DLQI (Dermatology Life Quality Index), and ADCT (Atopic Dermatitis Control Tool) were recorded up to 52 weeks of treatment. Results: The EASI, P-NRS, DLQI, and ADCT trends across phenotypes showed significant improvement in all phenotype subgroups. By week 16, classical and generalized lichenoid phenotypes showed the highest EASI improvements compared to the generalized inflammatory (75.0 vs. 45.5 [p < 0.001] and 79.3 vs. 45.5 [p < 0.001]), with most achieving EASI-75 (p < 0.001, χ2 = 25.96). By week 24, generalized lichenoid reached 100% EASI improvement, significantly outperforming other phenotypes. The highest EASI-75 rates were seen in classical, generalized lichenoid, and portrait/head and neck phenotypes (p = 0.016, χ2 = 13.85). No significant differences were observed at weeks 32, 40, or 52. Conclusions: Our results suggest that tralokinumab's durability and tolerability are consistent across the various phenotypes. The classical and generalized lichenoid were the fastest phenotypes to improve. However, given the uneven distribution of phenotypes and the gradual reduction in patient numbers over time, larger prospective studies are essential to confirm the observed trends.
Keywords: atopic dermatitis; tralokinumab.
Conflict of interest statement
S. Ferrucci has conflicts of interest with Amgen, Sanofi, Novartis, Lilly, LEO Pharma, Abbvie, Menarini. F. Barei has conflicts of interest with LEO Pharma and Almirall. M.B. Guanti has conflicts of interest with LEO Pharma, Abbvie, Sanofi, Menarini, Novartis, Cantabria, Lilly, Pfizer, and Firma. I. Trave has conflicts of interest with Abbvie, LEO Pharma, Galderma, and Novartis. E. Cozzani has conflicts of interest with Eli Lilly. M. Rossi has conflicts of interest with Abbvie, Sanofi, LEO Pharma, Pfizer, L’Oréal, and Almirall. E. Nettis has conflicts of interest with Sanofi, Abbvie, LEO Pharma, GSK, and AstraZeneca. K. Hansel has conflicts of interest with AbbVie, Almirall, Eli Lilly, LEO Pharma, Sanofi, and UCB. L. Stingeni has conflicts of interest with AbbVie, Almirall, Amgen, Bristol Meyer Squibb, Eli Lilly, Janssen, LEO Pharma, Novartis, and Sanofi. E. Pezzolo has conflicts of interest with Sanofi, LEO Pharma, Abbvie, and Pfizer. Novartis, Galderma, Janssen, Almirall, and Boehringer Ingelheim. F. Caroppo has conflicts of interest with Sanofi, LEO Pharma, Amgen, Novartis, and Abbvie. A. Belloni Fortina has conflicts of interest with Sanofi, LEO Pharma, Amgen, Novartis, Abbvie, Eli Lilly, Unifarco, and Almirall. G. Girolomoni has conflicts of interest with AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, LEO Pharma, Merck Serono, Novartis, Pfizer, Pierre Fabre, Samsung Bioepis, and Sanofi. S. Ribero has conflicts of interest with Sanofi, Abbvie, Almirall, UCB Pharma, Novartis, LEO Pharma, and Eli Lilly. Angelo Valerio Marzano has conflicts of interest with AbbVie, Boehringer Ingelheim, Novartis, Pfizer, Sanofi, and UCB. Anna Campanati has conflicts of interest with AbbVie, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, LEO Pharma, Novartis, Pfizer, Sanofi and Janssen, and UCB. The other authors have no conflicts of interest.
Figures

References
-
- Böhner A., Jargosch M., Müller N.S., Garzorz-Stark N., Pilz C., Lauffer F., Wang R., Roenneberg S., Zink A., Thomas J., et al. The neglected twin: Nummular eczema is a variant of atopic dermatitis with codominant TH2/TH17 immune response. J. Allergy Clin. Immunol. 2023;152:408–419. doi: 10.1016/j.jaci.2023.04.009. - DOI - PubMed
-
- Wollenberg A., Blauvelt A., Guttman-Yassky E., Worm M., Lynde C., Lacour J.P., Spelman L., Katoh N., Saeki H., Poulin Y., et al. ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br. J. Dermatol. 2021;184:437–449. doi: 10.1111/bjd.19574. - DOI - PMC - PubMed

