Synthesis, Molecular Simulation, DFT, and Kinetic Study of Imidazotriazole-Based Thiazolidinone as Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase Enzymes
- PMID: 40143192
- PMCID: PMC11944621
- DOI: 10.3390/ph18030415
Synthesis, Molecular Simulation, DFT, and Kinetic Study of Imidazotriazole-Based Thiazolidinone as Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase Enzymes
Abstract
Background: Alzheimer's disease is a complex and multifactorial brain disorder characterized by gradual memory impairment, cognitive disturbance, and severe dementia, and, ultimately, its progression leads to patient death. This research work presents the design, synthesis, and characterization of novel imidazotriazole-based thiazolidinone derivatives (1-14), displaying promising anti-Alzheimer's activity. Methods: These derivatives were synthesized by using 1H-imidazole-2-thiol as a starting reagent. Structural characterization was accomplished by 13C-NMR and 1H-NMR, while the molecular weight was confirmed by HREI-MS. These compounds were investigated for their anti-Alzheimer's potential under an in vitro analysis. Results: These compounds showed a significant to moderate biological potential against AChE and BChE in comparison to donepezil (IC50 = 8.50 µM and 8.90 µM against AChE and BuChE), used as a reference drug. Among these compounds, analog 10 with IC50 values of 6.70 µM and 7.10 µM against AChE and BuChE emerged as the lead compound of the series with promising biological efficacy against targeted enzymes. Molecular docking revealed the interactive nature of active ligands against target enzymes. These compounds were also assessed under dynamic conditions to examine the structural deviation and conformational changes in a protein complex structure. DFT calculations provided the relative stability and reactivity of the lead compounds. An ADMET analysis showed that these compounds have no toxicological profile. Conclusions: This research study paves the way for the further development and optimization of novel and selective imidazotriazole-based thiazolidinone inhibitors as potent anti-Alzheimer's agents.
Keywords: Alzheimer’s disease; MD simulations; molecular docking; pharmacophore modeling; triazole-thiazolidinone.
Conflict of interest statement
There are no conflicts of interest between the authors of the current manuscript.
Figures






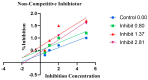


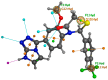




References
LinkOut - more resources
Full Text Sources
Miscellaneous

