Discovering New Tyrosinase Inhibitors by Using In Silico Modelling, Molecular Docking, and Molecular Dynamics
- PMID: 40143194
- PMCID: PMC11946302
- DOI: 10.3390/ph18030418
Discovering New Tyrosinase Inhibitors by Using In Silico Modelling, Molecular Docking, and Molecular Dynamics
Abstract
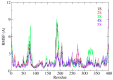
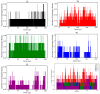
Background/Objectives: This study was used in silico modelling to search for potential tyrosinase protein inhibitors from a database of different core structures for IC50 prediction. Methods: Four machine learning algorithms and topographical descriptors were tested for model construction. Results: A model based on multiple linear regression was the most robust, with only six descriptors, and validated by the Tropsha test with statistical parameters R2 = 0.8687, Q2LOO = 0.8030, and Q2ext = 0.9151. From the screening of FDA-approved drugs and natural products, the pIC50 values for 15,424 structures were calculated. The applicability domain analysis covered 100% of the external dataset and 71.22% and 73.26% of the two screening datasets. Fifteen candidates with pIC50 above 7.6 were identified, with five structures proposed as potential tyrosinase enzyme inhibitors, which underwent ADME analysis. Conclusions: The molecular docking analysis was performed for the dataset used in the training-test process and for the fifteen structures from the screening dataset with potential pharmaceutical tyrosinase inhibition, followed by molecular dynamics studies for the top five candidates with the highest predicted pIC50 values. The new use of these five candidates in tyrosinase inhibition is highlighted based on their promising application in melanoma treatment.
Keywords: QSAR; melanoma; molecular docking; molecular dynamics; tyrosinase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
LinkOut - more resources
Full Text Sources

