rVSVΔG-ZEBOV-GP Vaccine Is Highly Immunogenic and Efficacious Across a Wide Dose Range in a Nonhuman Primate EBOV Challenge Model
- PMID: 40143273
- PMCID: PMC11945660
- DOI: 10.3390/v17030341
rVSVΔG-ZEBOV-GP Vaccine Is Highly Immunogenic and Efficacious Across a Wide Dose Range in a Nonhuman Primate EBOV Challenge Model
Abstract
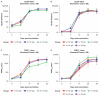
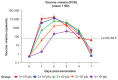
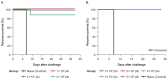
The recombinant vesicular stomatitis virus-Zaire Ebolavirus envelope glycoprotein vaccine (rVSVΔG-ZEBOV-GP) was highly effective against Ebola virus disease in a ring vaccination trial conducted during the 2014-2016 outbreak in Guinea and is licensed by regulatory agencies including US FDA, EMA, and prequalified by WHO. Vaccination studies in a nonhuman primate (NHP) model guided initial dose selection for clinical trial evaluation. We summarize two dose-ranging studies with the clinical-grade rVSVΔG-ZEBOV-GP vaccine candidate to assess the impact of dose level on immune responses and efficacy in an NHP Ebola virus (EBOV) challenge model. Forty-six cynomolgus macaques were vaccinated with a wide range of rVSVΔG-ZEBOV-GP doses and challenged 42 days later intramuscularly with 1000 pfu EBOV. Vaccination with rVSVΔG-ZEBOV-GP induced relatively high levels of EBOV-specific IgG and neutralizing antibodies, measured using the same validated assays as used in rVSVΔG-ZEBOV-GP clinical trials. Similar responses were observed across dose groups from 1 × 108 to 1 × 102 pfu. A single vaccination conferred 98% protection from lethal intramuscular EBOV challenge across all dose groups. These results demonstrate that robust antibody titers are induced in NHPs across a wide range of rVSVΔG-ZEBOV-GP vaccine doses, correlating with high levels of protection against death from EBOV challenge.
Keywords: ERVEBO; Ebolavirus; nonhuman primates; vaccine; vesicular stomatitis virus.
Conflict of interest statement
A.C.S., J.C.T., M.M.E.S., P.M.S., J.W.H., S.M. and S.A.K. declare no conflicts of interest. S.D., K.L., M.E., Z.C., are employees of Merck Sharp & Dohme L.L.C., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. J.K.S. and B.-A.G.C. were employees at the time of this study of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, at the time of this study, and may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. T.P.M. was employee of NewLink Genetics Corp.
Figures

References
-
- World Health Organization (WHO) Ebola Virus Disease. [(accessed on 8 November 2024)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/ebola-virus-disease.
-
- Thorson A.E., Deen G.F., Bernstein K.T., Liu W.J., Yamba F., Habib N., Sesay F.R., Gaillard P., Massaquoi T.A., McDonald S.L.R., et al. Persistence of Ebola virus in semen among Ebola virus disease survivors in Sierra Leone: A cohort study of frequency, duration, and risk factors. PLoS Med. 2021;18:e1003273. doi: 10.1371/journal.pmed.1003273. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention (CDC) Outbreak History. [(accessed on 8 November 2024)]; Available online: https://www.cdc.gov/ebola/outbreaks/index.html.
Publication types
MeSH terms
Substances
Grants and funding
- N/A/U.S Department of Health and Human Services (HHS)
- N/A/Administration for Strategic Preparedness and Response (ASPR)
- HDTRA1-15-C-0058/Defense Threat Reduction Agency
- HHSO100201700012C/Biomedical Advanced Research and Development Authority (BARDA)
- N/A/Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
LinkOut - more resources
Full Text Sources
Medical
Research Materials

