Oropouche Virus: Isolation and Ultrastructural Characterization from a Human Case Sample from Rio de Janeiro, Brazil, Using an In Vitro System
- PMID: 40143301
- PMCID: PMC11946457
- DOI: 10.3390/v17030373
Oropouche Virus: Isolation and Ultrastructural Characterization from a Human Case Sample from Rio de Janeiro, Brazil, Using an In Vitro System
Abstract
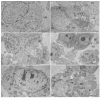
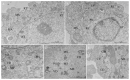
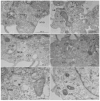
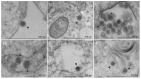
The Oropouche virus (OROV) is a segmented negative-sense RNA arbovirus member of the Peribunyaviridae family, associated with recurring epidemics of Oropouche fever in Central and South America. Since its identification in 1955, OROV has been responsible for outbreaks in both rural and urban areas, with transmission involving sylvatic and urban cycles. This study focuses on the characterization of an OROV isolate from a human clinical sample collected in the state of Rio de Janeiro, a non-endemic region in Brazil, highlighting ultrastructural and morphological aspects of the viral replicative cycle in Vero cells. OROV was isolated in Vero cell monolayers which, following viral inoculation, exhibited marked cytopathic effects (CPEs), mainly represented by changes in cell morphology, including membrane protrusions and vacuolization, as well as cell death. Studies by transmission electron microscopy (TEM) revealed significant ultrastructural changes, such as apoptosis, intense remodeling of membrane-bound organelles and signs of rough endoplasmic reticulum and mitochondrial stress. Additionally, the formation of specialized cytoplasmic vacuoles and intra- and extracellular vesicles emphasized trafficking and intercellular communication as essential mechanisms in OROV infection. RT-qPCR studies confirmed the production of viral progeny in high titers, corroborating the efficiency of this experimental model. These findings contribute to a better understanding of the cytopathogenic mechanisms of OROV infection and the contribution of cellular alterations in OROV morphogenesis.
Keywords: Oropouche virus; transmission electron microscopy; ultrastructural studies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Pinheiro F., Pinheiro M., Bensabath G., Causey O.R., Shope R.E. Epidemia de vírus Oropouche em Belém. Rev. Serv. Espec. Saude Publica. 1962;12:13–23.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

