Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability
- PMID: 40143329
- PMCID: PMC11945487
- DOI: 10.3390/v17030402
Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability
Abstract
The main protease (Mpro or 3CLpro or nsp5) of SARS-CoV-2 is crucial to the life cycle and pathogenesis of SARS-CoV-2, making it an attractive drug target to develop antivirals. This study employed the virtual screening of a few phytochemicals, and the resultant best compound, Scopoletin, was further investigated by a FRET-based enzymatic assay, revealing an experimental IC50 of 15.75 µM. The impact of Scopoletin on Mpro was further investigated by biophysical and MD simulation studies. Fluorescence spectroscopy identified a strong binding constant of 3.17 × 104 M⁻1 for Scopoletin binding to Mpro, as demonstrated by its effective fluorescence quenching of Mpro. Additionally, CD spectroscopy showed a significant reduction in the helical content of Mpro upon interaction with Scopoletin. The findings of thermodynamic measurements using isothermal titration calorimetry (ITC) supported the spectroscopic data, indicating a tight binding of Scopoletin to Mpro with a KA of 2.36 × 103 M-1. Similarly, interaction studies have also revealed that Scopoletin forms hydrogen bonds with the amino acids nearest to the active site, and this has been further supported by molecular dynamics simulation studies. These findings indicate that Scopoletin may be developed as a potential antiviral treatment for SARS-CoV-2 by targeting Mpro.
Keywords: FRET-based Mpro assay; Scopoletin; fluorescence quenching; isothermal titration calorimetry; main protease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


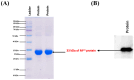


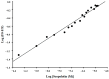
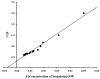


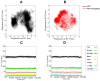
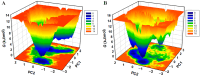
 boxes indicate change in heat energy in kJ mol−1 per injection of ligand to the protein.
boxes indicate change in heat energy in kJ mol−1 per injection of ligand to the protein.



References
-
- Dey J.K., Dey S.K. SARS-CoV-2 pandemic, COVID-19 case fatality rates and deaths per million population in India. J. Bioinform. Comput. Syst. Biol. 2020;2:5000110
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

