Belantamab Mafodotin Plus Proteasome Inhibition Efficacy Versus Comparators in Early Relapsed Myeloma: A Systematic Review and Network Meta-Analysis
- PMID: 40143674
- PMCID: PMC12067175
- DOI: 10.1002/ajh.27661
Belantamab Mafodotin Plus Proteasome Inhibition Efficacy Versus Comparators in Early Relapsed Myeloma: A Systematic Review and Network Meta-Analysis
Abstract
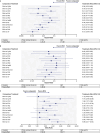
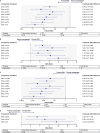
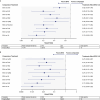
In the Phase 3 DREAMM-7 study of patients with relapsed/refractory multiple myeloma (RRMM) who received ≥ 1 prior therapy, belantamab mafodotin plus bortezomib and dexamethasone (BVd) demonstrated a progression-free survival (PFS) benefit versus daratumumab plus bortezomib and dexamethasone (DVd). This study aimed to indirectly compare the efficacy of BVd against alternative regimens in this patient population. A systematic literature review (SLR; December 2021-February 4, 2024) was performed to identify relevant efficacy data. Studies were selected based on the Population-Intervention-Comparators-Outcomes-Study design framework criteria and independently reviewed for inclusion in the network meta-analysis (NMA) if they had a connection to DREAMM-7 (approved in the US or EU, or likely to be a future DREAMM-7 comparator). Each trial had a common comparator arm, allowing for a connected network between the trials and linkage by shared treatments. The primary analysis was PFS in the intent-to-treat population from each study, and secondary analyses examined other endpoints. All endpoints were also evaluated in subgroups by lenalidomide-exposure, -refractoriness, and other patient characteristics. The SLR identified 12 comparator studies comprising 12 comparator regimens (each contained a proteasome inhibitor [bortezomib or carfilzomib] plus dexamethasone), all of which were included in the NMA with the DREAMM-7 study. BVd improved PFS versus all comparators, including daratumumab plus carfilzomib and dexamethasone, isatuximab plus carfilzomib and dexamethasone, and DVd. Overall survival was also improved by belantamab mafodotin plus bortezomib and dexamethasone over the other regimens. This study provides compelling evidence for belantamab mafodotin, plus bortezomib and dexamethasone, in early lines of treatment for RRMM.
Keywords: belantamab mafodotin combination; efficacy; meta‐analysis; relapsed/refractory multiple myeloma.
© 2025 GSK and The Author(s). American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
Joshua Richter reports consultancy fees from Janssen, Bristol Myers Squibb, Pfizer, Karyopharm, Sanofi, Takeda, Genentech, AbbVie, Regeneron and speaker bureau fees from Janssen, Bristol Myers Squibb, Sanofi, Adaptive Biotechnologies. Ajay Nooka has served on advisory boards and received honoraria from Adaptive Biotechnologies, Amgen, AstraZeneca, Bristol Myers Squibb, Cellectar Biosciences, GSK, Janssen, K36 Therapeutics, ONK Therapeutics, Pfizer, Sanofi, Sebia, and Takeda, has received institutional grant/research support from Aduro Biotech, Amgen, Arch Oncology, Bristol Myers Squibb, Cellectis, Genentech, GSK, Janssen, Karyopharm, Kite Pharma, Merck, Pfizer, and Takeda, and has received grant/research support for investigator‐initiated studies from Amgen, GSK, Janssen, Merck, and Takeda. Paula Rodríguez‐Otero reports consultancy fees from Bristol Myers Squibb, AbbVie, Roche, and Pfizer, has received honoraria from Bristol Myers Squibb, Janssen, Sanofi, GSK, Amgen, Regeneron, and Takeda, and is a member on the board of directors/speaker's bureau/advisory committee for Bristol Myers Squibb, Janssen, Sanofi, GSK, Amgen, Regeneron, Takeda, Kite Pharma, AbbVie, Oncopeptides, Pfizer, and GSK. Fredrik Schjesvold has received consultancy fees from AbbVie, Celgene, GSK, Janssen, Oncopeptides, Sanofi, and Takeda, has received research funding from Celgene, GSK, Janssen, Oncopeptides, Targovax, and Sanofi, and has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Daiichi Sankyo, GSK, Janssen, Novartis, Oncopeptides, Pfizer, Sanofi, SkylineDx, and Takeda. Eirini Katodritou served in a consulting or advisory role for Amgen and Janssen‐Cilag, received travel funding from Genesis Pharma and Takeda, received honoraria from Amgen, Genesis Pharma, Janssen‐Cilag, and Takeda, and received research funding from Amgen, Genesis Pharma, Janssen‐Cilag, and Takeda. Philippe Moreau served in a consulting or advisory role for Amgen, Celgene, GSK, Janssen, and Takeda, and received honoraria from Amgen, Celgene, GSK, Janssen‐Cilag, Novartis, and Takeda. Emily Combe, Marianne Scott, Leanne Cooper, and Indeg Sly are employees of FIECON. Nick Ballew, Jacopo Bitetti, Natalie Boytsov, Molly Purser, and Simon McNamara are employees of, and hold financial equities in, GSK.
Figures
References
-
- O'Donnell E. K., Shapiro Y. N., Yee A. J., et al., “Quality of Life, Psychological Distress, and Prognostic Perceptions in Patients With Multiple Myeloma,” Cancer 128, no. 10 (2022): 1996–2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

