Real-world experience with odevixibat in children with progressive familial intrahepatic cholestasis
- PMID: 40143945
- PMCID: PMC11937657
- DOI: 10.1016/j.jhepr.2024.101309
Real-world experience with odevixibat in children with progressive familial intrahepatic cholestasis
Abstract
Background & aims: A previously published trial demonstrated that odevixibat is effective in the treatment of cholestatic pruritus of children with progressive familial intrahepatic cholestasis (PFIC). Real-world experience is necessary to confirm the results of registration trials with selective eligibility criteria. We present our 'real-life experience' of the effectiveness and safety of odevixibat in patients with different PFIC subtypes.
Methods: We carried out a multicenter prospective study of patients with PFIC treated with odevixibat (40 or escalated to 120 μg/kg/day). Pruritus was assessed by 'Physician Global Impression of Symptom' at baseline and monthly up to 6 months. Serum bile acids (sBA) responders were patients who achieved a reduction in sBA levels ≥70% from baseline (or a value <70 μmol/L) after 6 months; pruritus responders were patients who reported improvement in their pruritus score.
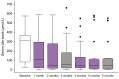
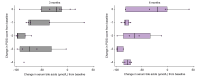
Results: In total, 24 patients (median age 6.6 years [3.7-12.1], male:female = 11/13) were enrolled; 16 (67%) had classic PFIC types (PFIC-1, 2; PFIC-2, 11; and PFIC-3, 3), whereas eight (33%) had rarer forms (PFIC-4, 5, PFIC-5, 1; PFIC-6, 1; and PFIC-9, 1). All had high sBA levels and 22/24 (92%) had pruritus. Four (17%) had associated comorbidities.After 6 months of treatment, sBA decreased from a median of 317.1 μmol/L (range 82.3-369.0 μmol/L) to 45.6 μmol/L (range 7.2-120 μmol/L; p <0.001); the mean change in pruritus score was -1.7. Overall, 75% of patients were sBA responders, 73% were pruritus responders, and 30% required dose escalation. Reduced pruritus correlated significantly with reduced sBA (p <0.05). A cut-off value of sBA >333.5 μmol/L increased the risk of no response to odevixibat by 17-fold (p <0.001). No serious adverse events were recorded.
Conclusions: Odevixibat is effective and safe in reducing sBA levels and improving pruritus in a real-life scenario in both patients with classic PFIC types and in those with other rarer subtypes. Dose escalation is required in some patients to improve the response to treatment.
Impact and implications: Published data on the use of odevixibat in a real-world scenario are lacking. We explored the effectiveness of odevixibat in a heterogenous cohort of children diagnosed with PFIC (including patients with classic as well as rarer types of PFIC, and with advanced liver disease and associated comorbidities). Our results demonstrate that odevixibat is effective for the treatment of cholestasis and pruritus in children with different PFIC subtypes in a real-life scenario. These results support the use of odevixibat in children with any type of PFICs, including those with different stages of liver disease and comorbidities.
Keywords: Bile acids; Children; Cholestasis; Jaundice; Odevixibat; PFIC; Pruritus; Real-life scenario.
© 2024 The Authors.
Conflict of interest statement
ADG, MS, MC, PC, GI, CM, EN are members of the advisory board for Ipsen and Mirum. AL is a consultant for Advanz Pharma, AlfaSigma, Takeda, Ipsen, and GSK; received speaker fees from Gilead, GSK, AbbVie, MSD, Advanz Pharma, AlfaSigma, GSK, and Incyte; and received travel support from Ipsen. FN: member of advisory board for Ipsen pharma. LD is a consultant or member of the advisory board of Mirum, Albireo/Ipsen, Alexion, Intercept/Advanz Pharma, Astra Zeneca, Vivet, Selecta, Spark, Tome, and Genespire. MF, SG, AM, FN, CZ, GC, AG declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures





References
LinkOut - more resources
Full Text Sources

