Diversity and inclusion in chronic hepatitis B randomised controlled trials: A systematic meta-epidemiological review
- PMID: 40143946
- PMCID: PMC11937669
- DOI: 10.1016/j.jhepr.2024.101324
Diversity and inclusion in chronic hepatitis B randomised controlled trials: A systematic meta-epidemiological review
Abstract
Background & aims: Chronic hepatitis B (CHB) affects global populations unequally, with variable prevalence and pathophysiology. Clinical trials must balance efficiency with adequate representation of the populations most likely to benefit from the interventions they test. We aimed to investigate diversity and inclusion in CHB trials.
Methods: We performed a meta-epidemiological study of randomised controlled trials recruiting people with CHB published in MEDLINE and Embase, January 2010 to July 2023. We extracted participant age, sex, country of recruitment, race and ethnicity, and hepatitis B genotype. We calculated proportions of trials reporting participant demographics and results by demographics (transparency). We compared participants proportionately to global populations affected by CHB of different demographics (representation), and examined demographic-based trial exclusion criteria.
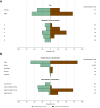
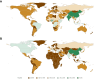
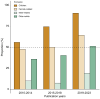
Results: Among 287 trials (81.9% single-country, 18.1% multinational) with 57,503 participants (median size: 102, IQR: 60-185), 97.9% tested drug interventions. Most trials reported participants' age distribution (285, 99.3%) and sex (278, 96.9%). However, only 37.3% (107) trials reported race and ethnicity, 84 (29.3%) reported genotype, and, among multinational trials, only 19 (36.5%) reported recruitment numbers by country. Less than 3% trials reported demography-stratified results. Female sex was under-represented (42.0% people with CHB, 28.7% trial participants). Geographic disparities between those affected by CHB and trial participation were marked for Africa (31.0% vs. 0.01%; under-representation), and Americas or Europe (5.7% vs. 14.0%; over-representation). Many trials had exclusion criteria based on age (71.4% children, 41.5% older adults) or sex-related (157, 54.7%), mostly excluding women who were pregnant, breastfeeding, or of reproduction potential.
Conclusions: Clinical trials for CHB are not inclusive of women and people in Africa. Researchers, funders, and publishers should actively consider diversity and inclusion of trials.
Impact and implications: Clinical trials must balance the need to recruit homogenous participants to efficiently measure an intervention's effectiveness, with the need to produce evidence that can be applied to the whole population affected by a disease. We found chronic hepatitis B (CHB) clinical trials often failed to report basic demographic characteristics of participants, and had under-representation of women and people living in Africa. Given varied disease pathophysiology and treatment needs among different groups, this suggest a mismatch of evidence generation compared with the populations needing treatment, whereby the benefits and harms of different interventions across populations are not being adequately studied. We suggest relevant stakeholders, including researchers, funders, and publishers of CHB clinical trials, should actively recruit under-represented populations, target interventions to those most at need, and either consider demographic factors in results reporting and analysis, or make data easily available for interrogation.
Keywords: Chronic hepatitis B; Diversity; Equity; Hepatitis B virus; Inclusion; Meta-epidemiology; Representation.
© 2025 The Authors.
Conflict of interest statement
There are no conflicts of interest to declare. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Seaman C.P., Luong P., Xiao Y., et al. A global investment case for hepatitis B elimination: a modelling study. Lancet Gastroenterol Hepatol. 2023;8:932–942. - PubMed
-
- Knottnerus J.A., Tugwell P. Heterogeneity and clinical reality. J Clin Epidemiol. 2013;66:809–811. - PubMed
-
- National Academies of Sciences . National Academies Press; Washington, DC: 2022. Engineering, and medicine; policy and global affairs; committee on women in science, engineering, and medicine; committee on improving the representation of women and underrepresented minorities in clinical trials and research. Why diverse representation in clinical research matters and the current state of representation within the clinical research ecosystem.
-
- Versavel S., Subasinghe A., Johnson K., et al. Diversity, equity, and inclusion in clinical trials: a practical guide from the perspective of a trial sponsor. Contemp Clin Trials. 2023;126 - PubMed
LinkOut - more resources
Full Text Sources

