The Impact of TP53-Induced Glycolysis and Apoptosis Regulator on Prognosis in Hepatocellular Carcinoma: Association with Tumor Microenvironment and Ferroptosis
- PMID: 40144470
- PMCID: PMC11936447
- DOI: 10.1159/000540180
The Impact of TP53-Induced Glycolysis and Apoptosis Regulator on Prognosis in Hepatocellular Carcinoma: Association with Tumor Microenvironment and Ferroptosis
Abstract
Introduction: TP53-induced glycolysis and apoptosis regulator (TIGAR) is a p53 target protein that has critical roles in glycolysis and redox balance. The reports about the effect of TIGAR on prognosis and its biological role in hepatocellular carcinoma (HCC) are limited.
Methods: A total of 386 patients with HCC who had undergone hepatic resection were enrolled. Immunohistochemical staining for TIGAR was performed. Additionally, the regulation of malignant activity and ferroptosis by TIGAR was investigated in vitro.
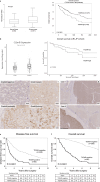
Results: Patients were divided into TIGAR-positive (n = 80, 20.7%) and -negative (n = 306, 79.3%) groups. TIGAR positivity was significantly correlated with lower albumin, higher α-fetoprotein/ des-gamma-carboxyprothrombin, larger tumor size/number of tumors, and greater proportions of BCLC staging C/single nodular type/poor differentiation/microscopic vascular invasion/microscopic intrahepatic metastasis. In multivariate analysis, TIGAR positivity was an independent prognostic factor (p < 0.0001). In addition, TIGAR positivity was significantly associated with a smaller number of cluster of differentiation (CD) 8-positive T cells (p = 0.0450), larger number of CD68-positive macrophages (p = 0.0058), larger number of programmed death-ligand 1-positive cases (p = 0.0002), and larger number of vessels that encapsulate tumor cluster-positive cases (p = 0.0004). In vitro, TIGAR knockdown decreased cell motility and induced ferroptosis. TIGAR knockdown inhibited the phosphorylation of adenosine monophosphate-activated protein kinase and acetyl-CoA carboxylase. Ferroptosis induced by TIGAR knockdown was inhibited by liproxstatin and baicalein treatment. The combination of TIGAR knockdown and lenvatinib further induced ferroptosis.
Conclusion: High expression of TIGAR impacted the clinical outcome of HCC patients and TIGAR was associated not only with tumor microenvironment but also with resistance to ferroptosis.
Keywords: Ferroptosis; Hepatocellular carcinoma; Lenvatinib; TIGAR.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflict of interest.
Figures




References
-
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–68. - PMC - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. - PubMed
-
- Iseda N, Itoh S, Yoshizumi T, Tomiyama T, Morinaga A, Shimagaki T, et al. . Lymphocyte-to-C-reactive protein ratio as a prognostic factor for hepatocellular carcinoma. Int J Clin Oncol. 2021;26(10):1890–900. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

