Adverse event reporting of faricimab: a disproportionality analysis of FDA adverse event reporting system (FAERS) database
- PMID: 40144657
- PMCID: PMC11936923
- DOI: 10.3389/fphar.2025.1521358
Adverse event reporting of faricimab: a disproportionality analysis of FDA adverse event reporting system (FAERS) database
Abstract
Background: Faricimab is the first and only bispecific antibody approved by the U.S. Food and Drug Administration (FDA) for intravitreal injection. Given its increasingly widespread use in retinal vascular diseases, understanding its adverse events (AEs) in real-world settings is crucial. This study employed the FDA Adverse Event Reporting System (FAERS) database to investigate potential safety concerns, with the aim of providing new insights for clinical practice.
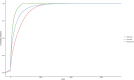
Methods: This study conducted a disproportionality analysis of adverse event data from the FAERS database, in which faricimab was identified as the primary suspect, covering the period from the first quarter of 2022 to the second quarter of 2024. To ensure the accuracy and reliability of the study, we employed four types of disproportionality analyses: the reporting odds ratio (ROR), proportional reporting ratio (PRR), multi-item gamma Poisson shrinker (MGPS), and Bayesian confidence propagation neural network (BCPNN). Additionally, the Weibull distribution was utilized to model the risk of adverse events over time.
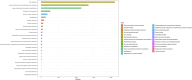
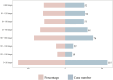
Results: A total of 2,735 adverse reaction reports, in which faricimab was identified as the primary suspect, were retrieved from the FAERS database. The analysis showed that faricimab-induced AEs occurred across 25 system organ classes (SOCs), with eye disorders meeting the positive threshold for all four algorithms. Significant AEs were mapped to preferred terms (PT), identifying the adverse reactions listed on the drug label: endophthalmitis, elevated intraocular pressure, cataract, retinal pigment epithelial tear, vitreous floaters, retinal vasculitis, retinal artery occlusion, and retinal vein occlusion. In addition to the AEs listed on the drug label, several previously unreported AEs were identified, including blindness, cerebral infarction, retinal hemorrhage, retinal occlusive vasculitis, glaucoma, dry eye, metamorphopsia, and unilateral blindness.
Conclusion: This study provided valuable evidence on the real-world safety of faricimab, suggesting that clinicians should place greater emphasis on monitoring its adverse effects during use.
Keywords: FAERS database; adverse events; disproportionality; faricimab; pharmacovigilance.
Copyright © 2025 He, Qiu, Lu, Xue, Liu and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

