Females with Fabry disease: an expert opinion on diagnosis, clinical management, current challenges and unmet needs
- PMID: 40144933
- PMCID: PMC11937019
- DOI: 10.3389/fcvm.2025.1536114
Females with Fabry disease: an expert opinion on diagnosis, clinical management, current challenges and unmet needs
Abstract
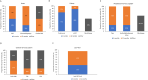
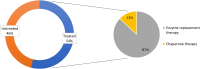
Females with Fabry disease (FD) often have a milder phenotype, later symptom onset, and slower disease progression than males, causing delayed diagnosis and undertreatment. A survey was conducted at nine Italian FD centers to evaluate routine management of females with FD; results were discussed at a meeting of eleven Italian specialists and recommendations developed. Of the 227 females managed by the physicians surveyed, 85% were diagnosed through family screening and 38.5% were symptomatic at presentation. Female patients usually underwent cardiac, renal, and neurologic monitoring, and measurement of plasma lyso-globotriaosylsphingosine (Gb3) levels at 6- or 12-month intervals. Treatment was initiated in 54%, mostly enzyme replacement therapy. Experts recommended screening all female relatives of index cases and evaluating all potentially affected organ systems. Diagnosis should be based on genetic analysis. Individualized monitoring of asymptomatic females must balance the need to detect organ damage while maintaining adherence. Treatment decisions should be based primarily on signs/symptoms of FD, but age, family screening results, GLA mutations, Gb3/lyso-Gb3 accumulation, and organ damage should be considered in asymptomatic females. More research on FD in females is needed and physicians should be aware of differences in the diagnosis, monitoring, and management of females vs. males with FD.
Keywords: Fabry disease; alpha-galactosidase A; enzyme replacement therapy; female; genetic testing; heterozygote.
© 2025 Tuttolomondo, Chimenti, Cianci, Gallieni, Lanzillo, La Russa, Limongelli, Mignani, Olivotto, Pieruzzi and Pisani.
Conflict of interest statement
AT received remuneration for participation in advisory boards on Fabry disease from Sanofi. CC received remuneration for participation in advisory boards on Fabry disease from Sanofi and Takeda. VC received remuneration for participation in advisory boards on Fabry disease from Sanofi Genzyme, Amicus, and Takeda. MG received remuneration for participation in advisory boards on Fabry disease and presentations in Fabry disease symposia from, and was sponsored for participating in Fabry disease meetings by, Sanofi Genzyme, Amicus, and Takeda; acted as a consultant for Becton Dickinson in clinical studies; was a member of a steering committee for Vifor in clinical studies; and received sponsorship for meeting participation and fees for presentations in symposia from Amicus, AstraZeneca, Baxter, Medtronic, Sanofi, and Vifor. CL received remuneration for participation in advisory boards on Fabry disease from Sanofi. ALR received remuneration for participation in advisory boards on Fabry disease from Sanofi. GL received remuneration for participation in advisory boards on Fabry disease from Sanofi. RM received sponsorship for meeting participation, fees for presentations in symposia, and remuneration for participation in advisory boards on Fabry disease from Sanofi Genzyme, Amicus, Chiesi, and Takeda. IO received remuneration for participation in advisory boards on Fabry disease from Sanofi, Shire Takeda, Amicus, and Chiesi; and received research support from BMS, Cytokinetics, Amicus, Genzyme, Shire Takeda, Menarini International, Chiesi, and Boston Scientific. FP received sponsorship for meeting participation for presentations in symposia and fees as a consultant in advisory boards on Fabry disease from Sanofi, Amicus, Chiesi, and Takeda. AP received sponsorship for meeting participation, fees for presentations in symposia, and remuneration for participation in advisory boards on Fabry disease from Sanofi Genzyme, Amicus, Chiesi, and Takeda. The handling editor FG declared a past collaboration with the authors IO, CC, CL, GL, RM, FP and AP.
Figures