Fostemsavir resistance in clinical context: a narrative review
- PMID: 40145022
- PMCID: PMC11938445
- DOI: 10.1177/20499361251325103
Fostemsavir resistance in clinical context: a narrative review
Abstract
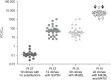
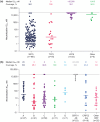
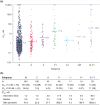
Fostemsavir, a prodrug of the first-in-class gp120-directed attachment inhibitor temsavir, is indicated in combination with other antiretrovirals for the treatment of multidrug-resistant HIV-1 in adults who are heavily treatment-experienced (HTE). Temsavir binds to HIV-1 gp120, close to the CD4 binding site, preventing the initial interaction of HIV-1 with CD4 on the host cell. Amino acid substitutions at four positions in gp120 have been identified as important determinants of viral susceptibility to temsavir (S375H/I/M/N/T/Y, M426L/P, M434I/K, M475I), with a fifth position (T202E) recently described. For most currently circulating group M HIV-1 subtypes, the prevalence of these resistance-associated polymorphisms (RAPs) is low. As with many other antiretrovirals, the impact of RAPs is modified by other changes in the target molecule. Different regions of gp120 interact to modify the temsavir binding pocket, with multiple amino acids playing a role in determining susceptibility. Extensive variability of HIV-1 gp120 means the susceptibility of clinical isolates to temsavir is also highly variable. Importantly, in vitro measurement of the susceptibility of clinical isolates to temsavir does not necessarily capture the range of susceptibilities of the heterogeneous mix of viruses generally present in each isolate. Due to these factors and limited phenotypic clinical data, thus far, no relevant phenotypic cutoff or genotypic algorithms have been derived that reliably predict response to fostemsavir-based therapy in individuals who are HTE; therefore, pre-treatment temsavir resistance testing may be of limited benefit. In the phase III BRIGHTE study, re-suppression after virologic failure was observed in some participants despite treatment-emergent genotypic and/or phenotypic evidence of reduced temsavir susceptibility, and substantial CD4+ T-cell count increases occurred even among participants with HIV-1 RNA ⩾40 copies/mL at Week 240. Clinical management of people who are HTE and experience virologic failure during treatment with fostemsavir-based regimens requires an individualized approach with consideration of potential benefits beyond virologic suppression.
Keywords: fostemsavir; heavily treatment-experienced; multidrug resistance.
© The Author(s), 2025.
Figures

References
-
- ViiV Healthcare. Rukobia (US prescribing information). Durham, NC: ViiV Healthcare, 2024.
-
- Kozal M, Aberg J, Pialoux G, et al.. Fostemsavir in adults with multidrug-resistant HIV-1 infection. N Engl J Med 2020; 382: 1232–1243. - PubMed
-
- Lalezari JP, Latiff GH, Brinson C, et al.. Safety and efficacy of the HIV-1 attachment inhibitor prodrug BMS-663068 in treatment-experienced individuals: 24 week results of AI438011, a phase 2b, randomised controlled trial. Lancet HIV 2015; 2: e427–e437. - PubMed
-
- Lataillade M, Lalezari JP, Kozal M, et al.. Safety and efficacy of the HIV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced individuals: week 96 results of the phase 3 BRIGHTE study. Lancet HIV 2020; 7: e740–e751. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

