Dengue Virus Dynamic and Persistence in Body Fluids of Infected Patients in Italy, 2018-2023
- PMID: 40145293
- PMCID: PMC11948169
- DOI: 10.1002/jmv.70322
Dengue Virus Dynamic and Persistence in Body Fluids of Infected Patients in Italy, 2018-2023
Abstract
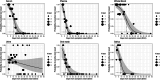
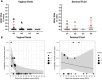
Dengue, a mosquito-borne disease caused by the dengue virus (DENV), is constantly expanding worldwide. We investigated the presence and persistence of DENV RNA in the bloodstream and other body fluids to describe the viral kinetics in the human host. We longitudinally collected serum (n = 118), plasma (n = 110), whole blood (n = 90), urine (n = 118), oral swabs (n = 68), saliva (n = 42), semen (n = 23), and vaginal fluids (n = 49) from 42 DENV patients. We measured DENV RNA for a median of 28 (range 1-63) days from symptom onset (DSO). We estimated the probability of viral detection applying a generalized linear model, and the duration of viremia using Monte Carlo-Markov Chain approach. In the bloodstream, the highest rate of positivity, levels of DENV RNA, and persistence were observed in whole blood. The estimated probability of a positive test dropped below 5% after 12.5, 20.7, and 35.4 DSO for plasma, serum, and whole blood, respectively. The average duration of viremia was estimated to be 19.9 DSO. Saliva and oral swabs showed 76.2% and 58.8% of DENV RNA positivity during the first week of symptoms while the longest persistence was observed in urine (39 DSO). DENV was revealed in 20% cervicovaginal (up to 11 DSO) and 30% seminal (up to 35 DSO) fluids. Whole blood represents the preferential specimen for dengue molecular detection and the correct estimation of viremia duration which have clear implications for onward transmission and public health countermeasures. Blood, urine, and oral samples can be assayed according to time from disease onset, severity, and screening purposes.
Keywords: arboviruses; dengue virus; laboratory diagnosis; viral shedding; viremia duration.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- CDC , “About Dengue,” CDC Centers for Disease Control and Prevention, (2024), https://www.cdc.gov/dengue/about/index.html.
-
- World Health Organization WHO , “Dengue ‐ Global Situation,” (2024), https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518.
-
- ECDC , “Local Transmission of Dengue Virus in Mainland EU/EEA, 2010‐Present,” (2024), https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disea....
-
- World Health Organization WHO , “Global Dengue Surveillance,” (2024), https://worldhealthorg.shinyapps.io/dengue_global/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

