Novel blood-based proteomic signatures across multiple neurodegenerative diseases
- PMID: 40145305
- PMCID: PMC11947754
- DOI: 10.1002/alz.70116
Novel blood-based proteomic signatures across multiple neurodegenerative diseases
Abstract
Introduction: Blood-based biomarkers have the potential to support early and accurate diagnoses of neurodegenerative diseases, which are sensitive to molecular pathology and are predictive of outcome. We evaluated a novel multiplex proteomic method in people with diverse neurodegenerative diseases.
Methods: Serum from people with Alzheimer's disease (N = 36), Lewy body dementia (N = 34), frontotemporal dementia (N = 36), and progressive supranuclear palsy (N = 36) and age-matched controls (N = 30) was analyzed with the nucleic acid linked immuno-sandwich assay (NULISA) central nervous system panel (≈ 120 analytes) and inflammation panel (250 analytes). Biomarkers were compared across groups and included as predictors of survival.
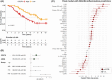
Results: The NULISA panels demonstrated high sensitivity and reliability for detecting multiple biomarkers across neurodegenerative disorders. There were condition-specific proteomic biomarkers, while neurofilament light chain, corticotropin-releasing hormone, CD276, and a data-driven inflammation pattern were significant transdiagnostic outcome predictors.
Discussion: The sensitive NULISA multiplex approach supports differential diagnosis and target identification, with prognostically informative dementia-related biomarkers.
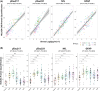
Highlights: We tested the novel technology nucleic acid linked immuno-sandwich assay (NULISA) in people with diverse neurodegenerative diseases, which demonstrated high sensitivity and reliability for detecting multiple biomarkers in serum samples. We compared the NULISA central nervous system serum results to single molecule array (Simoa) plasma assays for phosphorylated tau (p-tau)217, p-tau231, neurofilament light chain (NfL), and glial fibrillary acidic protein, finding strong correlations. Increased levels of serum NfL were identified across all patient groups and most elevated in the frontotemporal dementia (FTD) and progressive supranuclear palsy (PSP) cohorts, while p-tau epitopes were the most significant markers in patients with Alzheimer's disease (AD) and Lewy body dementia. Patients with FTD and PSP showed upregulation of many inflammation markers, compared to controls and patients with AD. We found condition-specific proteomic biomarkers, while NfL, corticotropin-releasing hormone, CD276, and data-driven immune signatures were significant transdiagnostic predictors of clinical outcomes (survival rates).
Keywords: blood‐based biomarkers; dementia; multiplex assays; non–Alzheimer's disease neurodegenerative diseases; nucleic acid linked immuno‐sandwich assay.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors have no conflicts of interest to report related to this work. Unrelated to this work, J.T.O. has received honoraria for work as DSMB chair or member for TauRx, Axon, Eisai, and Novo Nordisk; has acted as a consultant for Biogen and Roche; and has received research support from Alliance Medical and Merck. J.B.R. is a non‐remunerated trustee of the Guarantors of Brain, Darwin College, and the PSP Association (UK). He provides consultancy unrelated to the current work to Asceneuron, Astronautx, Astex, Curasen, CumulusNeuro, Wave, and SVHealth and has research grants from AZ‐Medimmune, Janssen, and Lilly as industry partners in the Dementias Platform UK. M.M. has acted as a consultant for Astex Pharmaceuticals. W.M. is an academic co‐founder and consultant to Trimtech Therapeutics and has a research grant funded by Takeda Pharmaceuticals. H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). A.H. has served as a consultant for Quanterix and been a paid panel member for Lilly. Author disclosures are available in the supporting information.
Figures



References
MeSH terms
Substances
Grants and funding
- ARUK-RADF2021A-010/Race Against Dementia Alzheimer's Research UK
- Progressive Supranuclear Palsy Association
- Dementias Platform UK
- Cambridge University Centre for Parkinson-Plus
- MC_UU_00030/14;MR/T033371/1/MRC_/Medical Research Council/United Kingdom
- #ALFGBG-71320/Swedish State Support for Clinical Research
- The Galen and Hilary Weston Foundation
- UK Dementia Research Institute
- 689-2024-01-Pipeline/CurePSP
- 101053962/HORIZON EUROPE European Innovation Council
- #2023-00356/Swedish Research Council
- #2022-01018/Swedish Research Council
- #2019-02397/Swedish Research Council
- BRC-1215-20014;NIHR203312/NIHR Cambridge Biomedical Research Centre
- 103838/WT_/Wellcome Trust/United Kingdom
- 220258/WT_/Wellcome Trust/United Kingdom
- PSP Association
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

