Does white matter and vascular injury from repetitive head impacts lead to a novel pattern on T2 FLAIR MRI? A hypothesis proposal and call for research
- PMID: 40145364
- PMCID: PMC11947747
- DOI: 10.1002/alz.70085
Does white matter and vascular injury from repetitive head impacts lead to a novel pattern on T2 FLAIR MRI? A hypothesis proposal and call for research
Abstract
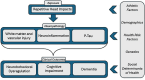
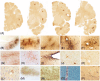
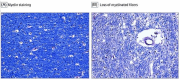
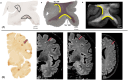
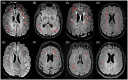
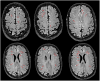
The goal of this paper is to introduce the hypothesis that white matter (WM) and vascular injury are long-term consequences of repetitive head impacts (RHI) that result in a novel T2 fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging pattern. A non-systematic literature review of autopsy and FLAIR studies of RHI-exposed adults was first conducted as a foundation for our hypothesis. A case series of RHI-exposed participants is presented to illustrate the unique FLAIR WM hyperintensities (WMH) pattern. Current literature shows a direct link between RHI and later-life WM/vascular neuropathologies, and that FLAIR WMH are associated with RHI, independent of modifiable vascular risk factors. Initial observations suggest a distinctive pattern of WMH in RHI-exposed participants, termed RHI-associated WMH (RHI-WMH). RHI-WMH defining features are as follows: (1) small, punctate, non-confluent, (2) spherical, and (3) proximal to the gray matter. Our hypothesis serves as a call for research to empirically validate RHI-WMH and clarify their biological and clinical correlates. HIGHLIGHTS: Repetitive head impacts (RHI) have been associated with later-life white matter (WM) and vascular neuropathologies. T2 FLAIR MRI of RHI-exposed participants reveals a potentially unique WM hyperintensity (WMH) pattern that is termed RHI-associated WMH (RHI-WMH). RHI-WMH are characterized as (1) small, punctate, and non-confluent, (2) spherical, and (3) proximal to the gray matter at an area anatomically susceptible to impact injury, such as the depths of the cortical sulci.
Keywords: FLAIR MRI; RHI‐WMH; chronic traumatic encephalopathy; contact and collision sports; fluid attenuated inversion recovery neuroimaging biomarkers; head trauma; neurodegenerative disease; repetitive head impacts; repetitive head impact‐associated white matter hyperintensities; traumatic brain injury; traumatic encephalopathy syndrome; white matter hyperintensities.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
G.D.R. has received grants from Genentech, the NIH, the Alzheimer's Association, and Rainwater Charitable Foundation outside the submitted work, and is an associate editor for
C.J.N. is a volunteer member of the Mackey–White Committee of the National Football League Players Association for which he receives travel support; an advisor and options‐holder with Oxeia Biopharmaceuticals, LLC, and StataDx; and has received travel support from the NFL, NFL Players Association, World Rugby, WWE, and AEW for lectures or conferences. C.J.N. has served as an expert witness in cases related to concussion and CTE and is compensated for speaking appearances and serving on the Players Advocacy Committee for the NFL Concussion Settlement. C.J.N. is employed by the Concussion Legacy Foundation, a 501(c)(3) non‐profit which receives charitable donations. R.A.S. is a member of the board of directors of King‐Devick Technologies, Inc. (Chicago, IL, USA), and he receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA), and consulting fees from Eisai. A.C.M. is a member of the Mackey–White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the National Institutes of Health and Department of Veteran Affairs and other funding from the Buoniconti Foundation and MacParkman Foundation during the conduct of the study. M.L.A. receives royalties from Oxford University Press Inc. and has received honorarium from the Michael J. Fox Foundation for services unrelated to this study. He also reports research support from Life Molecular Imaging Inc. and Rainwater Charitable Foundation Inc. The other authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures
References
-
- Montenigro PH, Corp DT, Stein TD, Cantu RC, Stern RA. Chronic traumatic encephalopathy: historical origins and current perspective. Annu Rev Clin Psychol. 2015;11:309‐330. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

