Pulmonary hypertension in Finland 2008-2020: A descriptive real-world cohort study (FINPAH)
- PMID: 40145556
- PMCID: PMC11935358
- DOI: 10.1016/j.jhlto.2024.100191
Pulmonary hypertension in Finland 2008-2020: A descriptive real-world cohort study (FINPAH)
Abstract
Background: To assess characteristics, risk group distribution, and prognosis of patients with pulmonary arterial hypertension (PAH) or chronic thromboembolic pulmonary hypertension (CTEPH) in Finland.
Methods: Clinical chart review of patients with PAH or CTEPH recorded between 2008 and 2019 and linkage to official mortality data.
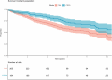
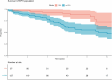
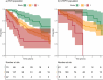
Results: We identified 627 patients, with 502 (80%) diagnosed after 2008, yielding an incidence of PAH and CTEPH of 4.0 and 2.9/million/year, respectively. The median time from symptoms to diagnosis was 1 year. Mean age at diagnosis of PAH patients (n = 268) was 57 years, 73% were women, 40% had idiopathic PAH, 28% associated with connective tissue diseases, and 15% with congenital heart disease, 9% had ≥3 cardiovascular comorbidities. At 1 year, 34%/34%/24%/8% were at the low/intermediate-low/intermediate-high/high Compera 2.0 risk classification groups. Survival was 91.3%, 74.8%, and 62.6% at 1, 3, and 5 years, respectively, with an improving trend over calendar time. Ten PAH patients had a lung transplant. PAH subtype, cardiac output, and the presence of ischemic heart disease or type 2 diabetes predicted survival.CTEPH patients (n = 189) were 63 years (mean) at diagnosis and 49% were women. Of the CTEPH patients, 29% underwent pulmonary endarterectomy (PEA) and 22% were treated with balloon pulmonary angioplasty. Survival was 94.6%, 87.2%, and 79.4% at 1, 3, and 5 years, respectively. PEA patients were younger, had fewer comorbidities, and had longer survival than non-PEA patients.
Conclusions: Incidence and survival of PAH and CTEPH patients in Finland were similar to previously presented data for other countries.
Keywords: chronic thromboembolic pulmonary hypertension; pulmonary arterial hypertension; real-world evidence; registry study; survival.
© 2024 International Society for Heart and Lung Transplantation.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Markku Pentikainen reports financial support was provided by State Research Funding (VTR) granted to Helsinki University Hospital. Markku Pentikainen reports financial support was provided by Janssen-Cilag OY. Piia Simonen reports financial support was provided by State Research Funding (VTR) granted to Helsinki University Hospital. Piia Simonen reports financial support was provided by Janssen-Cilag OY. Pauliina Leskela reports financial support was provided by Janssen-Cilag OY. Markku Pentikainen reports a relationship with Orion Corporation that includes equity or stocks. Markku Pentikainen reports a relationship with Janssen-Cilag OY that includes consulting or advisory, speaking and lecture fees, and travel reimbursement. Markku Pentikainen reports a relationship with MSD that includes consulting or advisory. Pauliina Leskela reports a relationship with Janssen-Cilag OY that includes speaking and lecture fees and travel reimbursement. Pauliina Leskela reports a relationship with Abbott that includes travel reimbursement. Pauliina Leskela reports a relationship with Medtronic that includes travel reimbursement. Terttu Harju reports a relationship with Janssen-Cilag OY that includes speaking and lecture fees and travel reimbursement. Terttu Harju reports a relationship with Nordic Infucare that includes travel reimbursement. Pertti Jaaskelainen reports a relationship with Janssen-Cilag OY that includes speaking and lecture fees and travel reimbursement. Airi Puhakka reports a relationship with Johnson & Johnson that includes equity or stocks. Airi Puhakka reports a relationship with Idorsia that includes equity or stocks. Airi Puhakka reports a relationship with Bittium that includes equity or stocks. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. We acknowledge Dr Sandra Hänninen, ESiOR Oy, for language proofreading. We acknowledge all participating patients and clinical staff. We acknowledge M. Kukkonen from HUS, L.-P. Lyytikäinen from TAYS, H. Lähdeaho from TAYS, P. Mankinen from ESiOR Oy, T. Vasankari from TYKS, H. Vihinen from TYKS, and M. Virtanen from TAYS for their contribution to data collection. This study was funded by Janssen-Cilag Oy, Espoo, Finland. M.P. and P.S. were funded by State Research Funding (VTR) granted to Helsinki University Hospital.
Figures

References
-
- Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG) Eur Heart J. 08 2022;43:3618–3731. - PubMed
-
- D'Alonzo G.E., Barst R.J., Ayres S.M., et al. Survival in patients with primary pulmonary hypertension. Ann Intern Med. 1991;115:343–349. - PubMed
-
- Rådegran G., Kjällström B., Ekmehag B., et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand Cardiovasc J. 2016;50:243–250. - PubMed
LinkOut - more resources
Full Text Sources

