Optogenetic control of Nodal signaling patterns
- PMID: 40145591
- PMCID: PMC12070070
- DOI: 10.1242/dev.204506
Optogenetic control of Nodal signaling patterns
Abstract
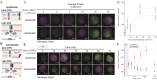
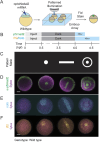
A crucial step in early embryogenesis is the establishment of spatial patterns of signaling activity. Tools to perturb morphogen signals with high resolution in space and time can help reveal how embryonic cells decode these signals to make appropriate fate decisions. Here, we present new optogenetic reagents and an experimental pipeline for creating designer Nodal signaling patterns in live zebrafish embryos. Nodal receptors were fused to the light-sensitive heterodimerizing pair Cry2/CIB1N, and the type II receptor was sequestered to the cytosol. The improved optoNodal2 reagents eliminate dark activity and improve response kinetics, without sacrificing dynamic range. We adapted an ultra-widefield microscopy platform for parallel light patterning in up to 36 embryos, and demonstrated precise spatial control over Nodal signaling activity and downstream gene expression. Patterned Nodal activation drove precisely controlled internalization of endodermal precursors. Furthermore, we used patterned illumination to generate synthetic signaling patterns in Nodal signaling mutants, rescuing several characteristic developmental defects. This study establishes an experimental toolkit for systematic exploration of Nodal signaling patterns in live embryos.
Keywords: Gastrulation; Mesendodermal patterning; Morphogen; Nodal signaling; Optogenetics; Zebrafish.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests A.F.S. serves on the scientific advisory board of Novartis. H.M.M. is a co-founder and scientific advisor for C16 Biosciences.
Figures


Update of
-
Optogenetic control of Nodal signaling patterns.bioRxiv [Preprint]. 2024 Apr 12:2024.04.11.588875. doi: 10.1101/2024.04.11.588875. bioRxiv. 2024. Update in: Development. 2025 May 1;152(9):dev204506. doi: 10.1242/dev.204506. PMID: 38645239 Free PMC article. Updated. Preprint.
References
-
- Beyer, H. M., Juillot, S., Herbst, K., Samodelov, S. L., Müller, K., Schamel, W. W., Römer, W., Schäfer, E., Nagy, F., Strähle, U.et al. (2015). Red light-regulated reversible nuclear localization of proteins in mammalian cells and zebrafish. ACS Synth. Biol. 4, 951-958. 10.1021/acssynbio.5b00004 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- K99 HD097297/HD/NICHD NIH HHS/United States
- GM056211/NH/NIH HHS/United States
- N00014-18-1-2859/U.S. Department of Defense
- R00 HD097297/HD/NICHD NIH HHS/United States
- 5K99HD097297/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- R37 GM056211/GM/NIGMS NIH HHS/United States
- R01 GM056211/GM/NIGMS NIH HHS/United States
- GM056211/GM/NIGMS NIH HHS/United States
- Fondecyt 11231198/Agencia Nacional de Investigación y Desarrollo Fondo Nacional de Desarrollo Científico y Tecnológico
- N00014-18-1-2859/National Science Foundation
- University of Pittsburgh
LinkOut - more resources
Full Text Sources

