N-Heterocyclic Carbenes: A Benchmark Study on their Singlet-Triplet Energy Gap as a Critical Molecular Descriptor
- PMID: 40145610
- PMCID: PMC12188164
- DOI: 10.1002/cphc.202500012
N-Heterocyclic Carbenes: A Benchmark Study on their Singlet-Triplet Energy Gap as a Critical Molecular Descriptor
Abstract
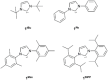
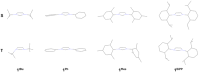
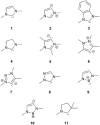
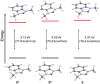
N-heterocyclic carbenes (NHCs) are used extensively in modern chemistry and materials science. The in-depth understanding of their electronic structure and their metal complexes remains an important topic of research and of experimental and theoretical interest. Herein, the adiabatic singlet-triplet gap as a superior, quantifiable critical descriptor, sensitive to the nature and the structural diversity of the NHCs, for a successful rationalization of experimental observations and computationally extracted trends is established. The choice is supported by a benchmark study on the electronic structures of NHCs, using high-level ab initio methods, that is, complete active space self-consistent field, n-electron valence second-order perturbation theory, multireference configuration interaction + singles + doubles, and domain-based local pair natural orbital-coupled cluster method with single-, double-, and perturbative triple excitations along with density functional theory methods such as BP86, M06, and M06-L, B3LYP, PBE0, TPSSh, CAM-B3LYP, and B2PLYP. In contrast to the adiabatic singlet-triplet (S-T) gap preferred as descriptor, the highest occupied molecular orbital-lowest unoccupied molecular orbital gap or the S-T vertical gap that has been used in the past occasionally leads to controversial results; some of these are critically discussed below. Extrapolation of these ideas to a group of copper-NHC complexes is also described.
Keywords: N ‐heterocyclic carbenes; ab initio; HOMO–LUMO gaps; density functional theories; singlet–triplet gaps.
© 2025 The Author(s). ChemPhysChem published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Igau A., Grutzmacher H., Baceiredo A., Bertrand G., J. Am. Chem. Soc. 1988, 110, 6463.
-
- Arduengo A. J., Harlow R. L., Kline M., J. Am. Chem. Soc. 1991, 113, 361.
-
- Cazin C. S. J., N‐Heterocyclic Carbenes In Transition Metal Catalysis and Organocatalysis, Springer Netherlands, Dordrecht: 2011.
-
- Díez‐González S., Marion N., Nolan S. P., Chem. Rev. 2009, 109, 3612. - PubMed
-
- Glorius F., N‐Heterocyclic Carbenes In Transition Metal Catalysis, Springer, Berlin, Heidelberg: 2007.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

