Current Understanding of Pathogenic Mechanisms and Disease Models of Citrin Deficiency
- PMID: 40145619
- PMCID: PMC11948450
- DOI: 10.1002/jimd.70021
Current Understanding of Pathogenic Mechanisms and Disease Models of Citrin Deficiency
Abstract
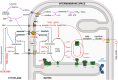
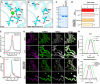
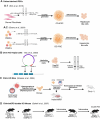
Citrin deficiency (CD) is a complex mitochondrial disease with three different age-related stages: neonatal intrahepatic cholestasis caused by CD (NICCD), failure to thrive and dyslipidemia caused by CD (FTTDCD), and type II citrullinemia (CTLN2), recently renamed adolescent and adult CD (AACD). While highly prevalent in the Asian population, CD is pan-ethnic and remains severely underdiagnosed. The disease is caused by the dysfunction or absence of the mitochondrial aspartate/glutamate carrier 2 (AGC2/SLC25A13), also known as citrin. Citrin deficiency results in a direct impairment of the malate-aspartate shuttle and the urea cycle, with expected knock-on effects on a multitude of other metabolic pathways, leading to a complicated pathophysiology. Here, we discuss our current knowledge of the molecular mechanism of substrate transport by citrin, including recent advances suggesting against its calcium regulation. We also discuss the different types of pathogenic variants found in CD patients and new insights into their pathogenic mechanisms. Additionally, we provide a summary and assessment of the efforts to develop preclinical models as well as treatments for the disease.
Keywords: citrin deficiency; disease models; mitochondrial transport; urea cycle disorders.
© 2025 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

