Elasticity-Driven Nanomechanical Interaction to Improve the Targeting Ability of Lipid Nanoparticles in the Malignant Tumor Microenvironment
- PMID: 40145669
- PMCID: PMC12245016
- DOI: 10.1002/advs.202502073
Elasticity-Driven Nanomechanical Interaction to Improve the Targeting Ability of Lipid Nanoparticles in the Malignant Tumor Microenvironment
Abstract
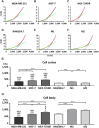
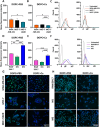
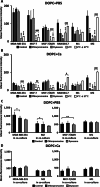
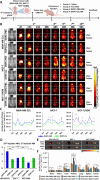
The mechanical elasticity of lipid nanoparticles (LNPs) is crucial to their pharmaceutical performance. This study investigates how the mechanical interactions between LNPs, target cells, and macrophages affect the internalization of LNPs into target cells at tumor sites. According to our bio-mechanical study, drug-resistant breast cancer cells are stiffer than sensitive ones, while invasive cells are softer; similarly, protumoral M2 macrophages are softer than M1 macrophages. Softer LNPs show increased cellular uptake in breast cancer cells and macrophages, with enhanced engulfment in invasive cells and M2 macrophages. Additionally, the presence of M2 macrophages promotes greater LNP internalization by cancer cells, facilitating the malignant and invasive nature of cancer cells. In addition, because breast cancer cells engulf LNPs via an energy-efficient fusion pathway but LNPs in macrophages undergo clathrin-mediated endocytosis, LNPs are internalized more into cancer cells but not into M2. In orthotopic tumor models, softer LNPs penetrate tumors quickly, enhancing suppression, whereas stiffer LNPs permeate slowly but show prolonged retention in stiffer tumors, supporting antitumor efficacy with repeated dosing. These findings underscore the importance of mechanical interactions between LNPs, target cells, and macrophages in optimizing LNP delivery systems, offering insights for more effective designs.
Keywords: atomic force microscopy; cellular uptake; lipid nanoparticles; mechanical property; permeation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Probing the Role of Lipid Nanoparticle Elasticity on mRNA Delivery to the Placenta.Nano Lett. 2025 Mar 26;25(12):4800-4808. doi: 10.1021/acs.nanolett.4c06241. Epub 2025 Mar 14. Nano Lett. 2025. PMID: 40084657
-
Lipid nanoparticle mediated mRNA delivery in cancer immunotherapy.Adv Immunol. 2025;166:37-75. doi: 10.1016/bs.ai.2025.02.001. Epub 2025 Mar 5. Adv Immunol. 2025. PMID: 40738545 Review.
-
EGFR-targeted ionizable lipid nanoparticles enhance in vivo mRNA delivery to the placenta.J Control Release. 2024 Jul;371:455-469. doi: 10.1016/j.jconrel.2024.05.036. Epub 2024 Jun 10. J Control Release. 2024. PMID: 38789090 Free PMC article.
-
Biomimetic cancer cell membrane engineered lipid nanoparticles for enhanced chemotherapy of homologous malignant tumor.BMC Cancer. 2025 Jul 1;25(1):1071. doi: 10.1186/s12885-025-14433-0. BMC Cancer. 2025. PMID: 40596990 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Polymeric and Polymer-Functionalized Drug Delivery Vectors: From Molecular Architecture and Elasticity to Cellular Uptake.Polymers (Basel). 2025 Aug 19;17(16):2243. doi: 10.3390/polym17162243. Polymers (Basel). 2025. PMID: 40871191 Free PMC article. Review.
References
-
- Kiio T. M., Park S., J. Pharm. Invest. 2021, 51, 35.
-
- Hui Y., Yi X., Hou F., Wibowo D., Zhang F., Zhao D., Gao H., Zhao C. X., ACS Nano 2019, 13, 7410. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
