Synergistic Modulating of Mitochondrial Transfer and Immune Microenvironment to Attenuate Discogenic Pain
- PMID: 40145798
- PMCID: PMC12199421
- DOI: 10.1002/advs.202500128
Synergistic Modulating of Mitochondrial Transfer and Immune Microenvironment to Attenuate Discogenic Pain
Abstract
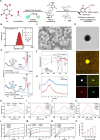
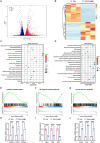
Discogenic pain, caused by intervertebral disc degeneration (IVDD), is a prevalent and challenging condition to treat effectively. Macrophage infiltration with neural ectopic in-growth resulting from structural disturbances within the intervertebral disc (IVD) is a major cause of discogenic pain. This work systematically reveals how nanoparticles can synergistically regulate the immune microenvironment and mitochondrial communication to attenuate discogenic pain. The antioxidant metal-polyphenol nanoparticle system can sequentially regulate macrophage phenotype and mitochondrial delivery efficiency. This strategy circumvents the necessity for mitochondrial isolation and preservation techniques that are typically required in conventional mitochondrial transplantation procedures. Furthermore, it facilitates the effective and sustained delivery of mitochondria to damaged cells. In vivo, this nanoparticle formulation effectively preserves the IVD height, maintains the structural integrity of the nucleus pulposus (NP), and restores pain thresholds. Thus, this nanoplatform offers an effective approach to traditional surgical treatments for discogenic pain, with significant potential for clinical application.
Keywords: discogenic pain; macrophage; mitochondrial transfer; nanoparticles.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Hartvigsen J., Hancock M. J., Kongsted A., Louw Q., Ferreira M. L., Genevay S., Hoy D., Karppinen J., Pransky G., Sieper J., Smeets R. J., Underwood M., Lancet 2018, 391, 2356. - PubMed
MeSH terms
Grants and funding
- ZY2022010/Wenzhou Major Scientific and Technological Innovation Project
- 82272555/National Natural Science Foundation of China
- 82472488/National Natural Science Foundation of China
- 2247020672/National Natural Science Foundation of China
- WIBEK181006/Research Center of Clinical Functional Materials and Diagnosis & Treatment Devices of Zhejiang Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
