Preparation of Thermally and Photochemically Immobilized N-type Conjugated Polymer Films via Quantitative Backbone Editing
- PMID: 40145877
- PMCID: PMC12124454
- DOI: 10.1002/anie.202505608
Preparation of Thermally and Photochemically Immobilized N-type Conjugated Polymer Films via Quantitative Backbone Editing
Abstract
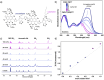
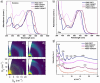
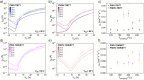
We report a series of n-type conjugated polymers based on PNDI-TfBTT and PNDIV-TfBTT backbones constructed from electron-deficient naphthalene diimide (NDI) and fluorinated benzothiadiazole (fBT) units, with PNDIV-TfBTT incorporating a vinylene spacer. Quantitative postpolymerization modification (PPM) via nucleophilic substitution replaced the fBT fluorine with thioether side chains, optionally containing azide groups. Thioether substitution improved solubility, while subtly changing the ordering of polymer films. Azide incorporation enabled both thermal and photochemical crosslinking, yielding insoluble and immobile films that retained good electron transport; although UV crosslinking initially reduced mobility, subsequent thermal annealing largely restored crystallinity and performance. This work underscores the utility of precise backbone editing to fine-tune the electronic and morphological properties of n-type polymers, offering new avenues for the fabrication of stable, patterned active layers in advanced organic electronic devices.
Keywords: Backbone editing; Conjugated polymer; Cross‐linking; N‐type material; Postpolymerization functionalization.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

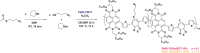

References
-
- Wei Q., Ge Z., Voit B., Macromol. Rapid Commun. 2019, 40, 1800570. - PubMed
-
- Grimsdale A. C., Leok Chan K., Martin R. E., Jokisz P. G., Holmes A. B., Chem. Rev. 2009, 109, 897–1091. - PubMed
-
- Woo J. Y., Park M.‐H., Jeong S.‐H., Kim Y.‐H., Kim B., Lee T.‐W., Han T.‐H., Adv. Mater. 2023, 35, 2207454. - PubMed
-
- Ling H., Liu S., Zheng Z., Yan F., Small Methods 2018, 2, 1800070.
-
- Zhang G., Lin F. R., Qi F., Heumüller T., Distler A., Egelhaaf H.‐J., Li N., Chow P. C. Y., Brabec C. J., Jen A. K.‐Y., Yip H.‐L., Chem. Rev. 2022, 122, 14180–14274. - PubMed
Grants and funding
- EP/V048686/1/Engineering and Physical Science Research Council
- EP/T028513/1/Engineering and Physical Science Research Council
- Royal Society and Wolfson Foundation
- OSR-2020-CRG8-4095/King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR)
- PID2021-126243NB-I00/MICINN
LinkOut - more resources
Full Text Sources

