Identification of Early-Stage Breast Cancer with a Minimal Risk of Recurrence by the Breast Cancer Index
- PMID: 40145943
- PMCID: PMC12130797
- DOI: 10.1158/1078-0432.CCR-24-3836
Identification of Early-Stage Breast Cancer with a Minimal Risk of Recurrence by the Breast Cancer Index
Abstract
Purpose: This study assessed the prognostic ability of the breast cancer index (BCI) to identify patients at a minimal risk (<5%) of 10-year distant recurrence (DR) who are unlikely to benefit from adjuvant endocrine therapy.
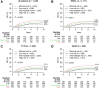
Experimental design: This prospective translational study included postmenopausal patients with early-stage, hormone receptor-positive N0 breast cancer from the Stockholm (STO-3) trial who underwent surgery alone ("untreated") or surgery plus adjuvant tamoxifen ("treated") and from the Netherlands Cancer Registry (surgery alone). The primary endpoint was time to DR. An adjusted BCI model with an additional cutpoint was developed, which stratified patients into four prognostic risk groups.
Results: Across cohorts, 16% to 22% of patients were classified as minimal risk of 10-year DR. In the Stockholm untreated cohort (n = 283), risks in the minimal-, low-, intermediate-, and high-risk groups were 2.3%, 15.5% [hazard ratio, 4.71 (95% confidence interval, 1.09-20.29) vs. minimal risk], 19.8% [6.97 (1.61-30.18)], and 35.9% [13.21 (3.07-56.76)], respectively (P < 0.001). In the Stockholm treated cohort (n = 317), risks were 4.3%, 5.0% [1.16 (0.35-3.85)], 11.7% [2.45 (0.74-8.14)], and 21.1% [5.27 (1.72-16.16); P < 0.001]. In the Netherlands Cancer Registry cohort (n = 1245), risks were 4.5%, 7.5% [subdistribution hazard ratio, 1.67 (95% confidence interval, 0.81-3.45)], 10.3% [2.40 (1.14-5.03)], and 13.1% [3.13 (1.50-6.55); P = 0.005]. BCI risk scores provided additional independent information over standard prognostic factors (likelihood ratio, χ2 = 7.98; P = 0.004).
Conclusions: The adjusted BCI model identified women with early-stage, hormone receptor-positive N0 breast cancer at a minimal risk of DR who may consider de-escalating adjuvant endocrine therapy.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
M. F. Jilderda reports grants from Hologic/Biotheranostics during the conduct of the study. Y. Zhang reports a patent for the biomarker analyzed in this work pending and issued. V. Rebattu reports grants from Hologic/Biotheranostics during the conduct of the study. O. Stål reports grants from The Swedish Cancer Society outside the submitted work. A.K.L. Anderson reports other support from Hologic outside the submitted work. K. Treuner reports a patent for BCI pending. G.-J. Liefers reports other support from Hologic during the conduct of the study. No disclosures were reported by the other authors.
Figures


References
-
- Early Breast Cancer Trialists' Collaborative Group EBCTCG; Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84. - PMC - PubMed
-
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998;351:1451–67. - PubMed
-
- National Comprehensive Cancer Network (NCCN) . NCCN clinical practice guidelines in oncology (NCCN guidelines). Breast cancer. Version 4.2024; 2024. - PubMed
-
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M; Arimidex Tamoxifen Alone or in Combination ATAC Trialists’ Group . Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008;9:45–53. - PubMed
-
- Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, et al. ; Breast International Group BIG 1-98 Collaborative Group . A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005;353:2747–57. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

