Development and psychometric evaluation of a patient-reported symptom index for patients with non-muscle invasive bladder cancer: the NMIBC-SI
- PMID: 40146452
- PMCID: PMC11950540
- DOI: 10.1186/s41687-025-00864-7
Development and psychometric evaluation of a patient-reported symptom index for patients with non-muscle invasive bladder cancer: the NMIBC-SI
Abstract
Background and objective: Non-muscle invasive bladder cancer (NMIBC) is a chronic condition requiring frequent follow-up with endoscopic examinations, tumour resections and intravesical treatments. In this clinical context, patient-reported outcomes (PROs) have enormous potential to inform treatment assessment and recommendations for NMIBC. We aimed to develop and evaluate a patient-reported NMIBC Symptom Index (NMIBC-SI) to facilitate clinical research and enhance care.
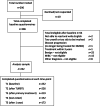
Methods: NMIBC-SI items were developed based on existing literature and qualitative interviews with patients and clinicians, and evaluated in two field tests: item reduction, using NMIBC-SI data from 220 patients on active treatment from nine Australian centres; reliability and validity evaluation of item-reduced version using NMIBC-SI data from 232 patients from five countries.
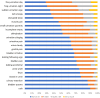
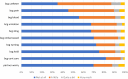
Results: NMIBC-SI assesses disease and treatment-related symptom burden and two treatment-specific side-effects (cystoscopy, intravesical BCG/Chemotherapy). Composite analysis supported a single composite model including core symptom and cystoscopy index items (Intravesical index items were not tested due to small sample). Test-retest reliability was strong (range 0.894-0.91). As expected, the NMIBC-SI was able to discriminate between no treatment and any treatment groups, and no treatment and chemo/BCG groups, providing evidence towards validity.
Conclusions and clinical implications: NMIBC-SI assesses patients' self-reported symptom burden and can be used to evaluate NMIBC treatments from the perspective of patients. The NMIBC-SI is acceptable to patients and has evidence for reliability and validity. Future validation work with patients with greater symptom burden is warranted.
Keywords: Early bladder cancer; Measurement properties; Non-muscle invasive bladder cancer; Patient-reported outcome; Patient-reported outcome measure; Symptom benefit; Symptom burden; Symptom index.
Plain language summary
Patients with NMIBC require long-term monitoring with regular endoscopic examinations and various treatments. This research describes the development of the NMIBC-SI, a patient-completed questionnaire that assesses the impact of those treatments on health outcomes important to patients. The NMIBC-SI can be used in research and in the clinic to improve communication and treatment decisions.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Northern Sydney Local Health District Human Research Ethics Committee on 26 October 2016 (HREC/16/HAWKE/329). Written informed consent was obtained from all individual participants included in the study. Consent included publication of results from this study in the form of anonymised survey data. Consent for publication: Not applicable. Clinical trial registration: ClinicalTrials.gov NCT03091764, 27 March 2017. http://clinicaltrials.gov/ct2/showNCT03091764 . Competing interests: The co-authors have disclosed the following as financial interests, relationships, and affiliations relevant to the subject matter discussed in the manuscript: SAB is a consultant for Ferring, FerGene, Artara, and Prokarium. SS is an unpaid board director of ANZUP trials group. Has been an adviser/speaker for Mundipharma, Ipsen, Abbvie and MSD (with honoraria donated directly into departmental research funds) and a paid adviser to Janssen. NB is an unpaid board director of ANZUP trials group. BK is a consultant for Ferring Pharmaceuticals Inc and Investigator clinical trial, Pacific Edge. MP is an advisor/speaker for Jannsen, MSD, Mundipharma, Astra Zenaca. The remaining authors declare that they have no competing interests.
Figures
References
-
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al (2024) Global Cancer Observatory: Cancer Today. https://gco.iarc.who.int/today. Accessed 06 March 2024
-
- Heney NM (1992) Natural history of superficial bladder cancer. Prognostic features and long-term disease course. Urol Clin North Am 19(3):429–433 - PubMed
-
- Sylvester RJ, Rodríguez O, Hernández V, Turturica D, Bauerová L, Bruins HM et al (2021) European association of urology (EAU) prognostic factor risk groups for Non–muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: An update from the EAU NMIBC guidelines panel. Eur Urol 79(4):480–488. 10.1016/j.eururo.2020.12.033 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous