Protocol for biomimetic tumoroid models by plastic compression using centrifugation
- PMID: 40146777
- PMCID: PMC11985085
- DOI: 10.1016/j.xpro.2025.103718
Protocol for biomimetic tumoroid models by plastic compression using centrifugation
Abstract
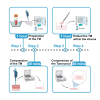
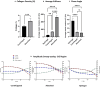
Here, we present a protocol for engineering biomimetic tumoroid models by plastic compression using centrifugation. We describe steps for generating multi-compartment tumor-stroma models by mixing cells into a collagen hydrogel crosslinked at 37°C and centrifuging the hydrogel. We then detail procedures for generating compartmentalized models and encapsulating the final layered hydrogel containing a 96-well tumor mass in a 24-well stroma. This protocol increases collagen density and improves mechanical properties of collagen hydrogels.
Keywords: Cancer; Cell-based Assays; Tissue Engineering.
Crown Copyright © 2025. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

