Cholesterol-dependent enzyme activity of human TSPO1
- PMID: 40146857
- PMCID: PMC12002307
- DOI: 10.1073/pnas.2323045122
Cholesterol-dependent enzyme activity of human TSPO1
Abstract
The amino acid sequence of the tryptophan-rich sensory proteins (TSPO) is substantially conserved throughout all kingdoms of life. Human mitochondrial TSPO1 (HsTSPO1) binds to porphyrins and steroids, although its interactions with these molecules remains unknown. HsTSPO1 is associated with numerous physiological and pathological disorders, but the underlying molecular mechanisms are unknown. Here, we disclose the finding of human mitochondrial TSPO as a cholesterol-dependent protoporphyrin IX oxygenase. The results of our biochemical characterization are consistent with structural data and evolutionary analysis. The dependence of HsTSPO1 activity on cholesterol may be the result of the coevolution of this membrane protein with the membrane system. Our study provides a molecular foundation for comprehending the various roles played by mitochondrial TSPO in normal physiological and pathological situations.
Keywords: NCMN; TSPO; bilindigin; cholesterol; protoporphyrin IX.
Conflict of interest statement
Competing interests statement:Y.G. has patents pending related to the NCMN system, PpIX-2, Billindigin and the bioactive peripheral benzodiazepine receptor. The NCMN system stands for Native Cell Membrane Nanoparticles system. It is a detergent-free system developed for membrane protein research. PpIX-2 is a derivative molecule of protoporphyrin IX that exhibits enhanced water solubility. Bilindigin is an oxidized product of protoporphyrin IX catalyzed by TSPO proteins. The bioactive peripheral benzodiazepine receptor (HsTSPO1) can be well stabilized via the NCMN system and holds potential for applications in fundamental research, pharmacological development, and other industrial uses. T.K.H.T. shares a pending patent related to PpIX-2. Y.G. and T.K.H.T co-invented CHEAPS, a cholesterol derivative molecule with a balanced hydrophobicity and hydrophilicity (marketed by Anatrace Inc., Catalog # CH240). W.Q. shares a pending patent related to the bioactive peripheral benzodiazepine receptor. Y.G. and W.Q. are the founders of a startup, NCMNtech LLC.
Figures

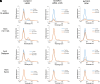
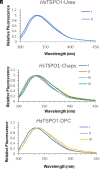
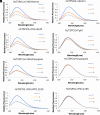
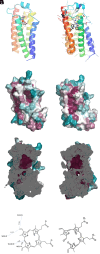
References
-
- Papadopoulos V., et al. , Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 27, 402–409 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

