Nuclear hormone receptors control fundamental processes of human fetal neurodevelopment: Basis for endocrine disruption assessment
- PMID: 40147140
- PMCID: PMC12127433
- DOI: 10.1016/j.envint.2025.109400
Nuclear hormone receptors control fundamental processes of human fetal neurodevelopment: Basis for endocrine disruption assessment
Abstract
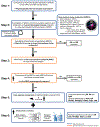
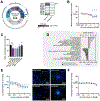
Despite growing awareness of endocrine disrupting chemicals (EDCs), knowledge gaps remain regarding their effects on human brain development. EDC risk assessment focuses primarily on EATS modalities (estrogens, androgens, thyroid hormones, and steroidogenesis), overlooking the broader range of hormone receptors expressed in the developing brain. This limits the evaluation of chemicals for their potential to cause endocrine disruption-mediated developmental neurotoxicity (ED-DNT). The Neurosphere Assay, an in vitro test method for developmental neurotoxicity (DNT) evaluation, is an integral component of the DNT in vitro testing battery, which has been used to screen a broad domain of environmental chemicals. Here, we define the endocrine-related applicability domain of the Neurosphere Assay by assessing the impact and specificity of 14 hormone receptors on seven key neurodevelopmental processes (KNDPs), neural progenitor cell (NPC) proliferation, migration of radial glia, neurons, and oligodendrocytes, neurite outgrowth, and differentiation of neurons and oligodendrocytes. Comparative analyses in human and rat NPCs of both sexes revealed species- and sex-specific responses. Mechanistic insights were obtained through RNA sequencing and agonist/antagonist co-exposures. Most receptor agonists modulated KNDPs at concentrations in the range of physiologically relevant hormone concentrations. Phenotypic effects induced by glucocorticoid receptor (GR), liver X receptor (LXR), peroxisome proliferator-activated receptor beta/delta (PPARβδ), retinoic acid receptor (RAR) and retinoid X receptor (RXR) activation were counteracted by receptor antagonists, confirming specificity. Transcriptomics highlighted receptor crosstalk and the involvement of conserved developmental pathways (e.g. Notch and Wnt). Species comparisons identified limited concordance in hormone receptor-regulated KNDPs between human and rat NPCs. This study presents novel findings on cellular and molecular hormone actions in human fetal NPCs, highlights major species differences, and illustrates the Neurosphere Assay's relevance for detecting endocrine MoAs, supporting its application in human-based ED-DNT risk assessment.
Keywords: Developmental neurotoxicity; EDC; Endocrine disruption; Neural progenitor cells; Neurons; New approach methodologies; Oligodendrocytes.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Katharina Koch reports financial support was provided by European Commission. Ellen Fritsche reports financial support was provided by European Commission. Joerdis Klose reports financial support was provided by FOKO (Forschungskommision of the medical faculty of the Heinrich-Heine-University). Ellen Fritsche reports financial support was provided by FOKO (Forschungskommision of the medical faculty of the Heinrich-Heine-University). Arif Doenmez reports financial support was provided by European Commission. Stefan Masjosthusmann reports financial support was provided by German Ministry of Education and Research (BMBF). Katharina Koch reports a relationship with DNTOX GmbH that includes: employment and equity or stocks. Joerdis Klose reports a relationship with DNTOX GmbH that includes: employment and equity or stocks. Arif Doenmez reports a relationship with DNTOX GmbH that includes: employment and equity or stocks. Ellen Fritsche reports a relationship with DNTOX GmbH that includes: employment and equity or stocks. The data for this manuscript was generated solely at the IUF prior to the foundation of the DNTOX GmbH. The funders of DNTOX had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM, 2013. Glucocorticoid-Related Molecular Signaling Pathways Regulating Hippocampal Neurogenesis. Neuropsychopharmacology 38, 872–883. 10.1038/npp.2012.253. - DOI - PMC - PubMed
-
- Araki A, Mitsui T, Goudarzi H, Nakajima T, Miyashita C, Itoh S, Sasaki S, Cho K, Moriya K, Shinohara N, Nonomura K, Kishi R, 2017. Prenatal di(2-ethylhexyl) phthalate exposure and disruption of adrenal androgens and glucocorticoids levels in cord blood: The Hokkaido Study. Sci. Total Environ 581–582, 297–304. 10.1016/J.SCITOTENV.2016.12.124. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

