Rheological transition driven by matrix makes cancer spheroids resilient under confinement
- PMID: 40147946
- PMCID: PMC11949850
- DOI: 10.26508/lsa.202402601
Rheological transition driven by matrix makes cancer spheroids resilient under confinement
Abstract
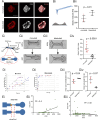
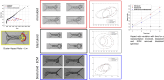
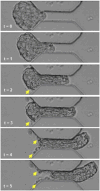
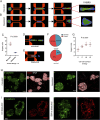
Cancer metastasis through confining peritoneal microenvironments is mediated by spheroids: clusters of disseminated cells. Ovarian cancer spheroids are frequently cavitated; such blastuloid morphologies possess an outer ECM coat. We investigated the effects of these spheroidal morphological traits on their mechanical integrity. Atomic force microscopy showed blastuloids were elastic compared with their prefiguring lumenless moruloid counterparts. Moruloids flowed through microfluidic setups mimicking peritoneal confinement, exhibited asymmetric cell flows during entry, were frequently disintegrated, and showed an incomplete and slow shape recovery upon exit. In contrast, blastuloids exhibited size-uncorrelated transit kinetics, rapid and efficient shape recovery upon exit, symmetric cell flows, and lesser disintegration. Blastuloid ECM debridement phenocopied moruloid traits including lumen loss and greater disintegration. Multiscale computer simulations predicted that higher intercellular adhesion and dynamical lumen make blastuloids resilient. Blastuloids showed higher E-cadherin expression, and their ECM removal decreased membrane E-cadherin localization. E-cadherin knockdown also decreased lumen formation and increased spheroid disintegration. Thus, the spheroidal ECM drives its transition from a labile viscoplastic to a resilient elastic phenotype, facilitating their survival within spatially constrained peritoneal flows.
© 2025 Dutt et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
-
- Goyeneche A, Lisio MA, Fu L, Srinivasan R, Valdez Capuccino J, Gao ZH, Telleria C (2020) The capacity of high-grade serous ovarian cancer cells to form multicellular structures spontaneously along disease progression correlates with their orthotopic tumorigenicity in immunosuppressed mice. Cancers 12: 699. 10.3390/cancers12030699 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
