Mass distribution of azithromycin and child mortality among underweight infants in rural Niger: a subgroup analysis of the AVENIR cluster-randomised trial
- PMID: 40147984
- PMCID: PMC11956350
- DOI: 10.1136/bmjopen-2024-097916
Mass distribution of azithromycin and child mortality among underweight infants in rural Niger: a subgroup analysis of the AVENIR cluster-randomised trial
Abstract
Objective: Azithromycin has been shown to reduce all-cause child mortality. This subgroup analysis investigates azithromycin's mortality impact by underweight status using Azithromycine pour la Vie des Enfants au Niger: Implementation et Recherche (AVENIR) trial data.
Design: The AVENIR trial randomised communities into three arms: azithromycin for children aged 1-59 months, azithromycin for infants aged 1-11 months or placebo. Weight-for-age z-score was used to categorise children into subgroups of either moderate to severe underweight or not and severe underweight or not.
Setting: 2880 communities with a population of less than 2500 people in the Dosso and Tahoua regions of Niger that participated in the AVENIR trial were included.
Participants: 97 572 children aged 1-59 months who had weight captured during at least one census participated.
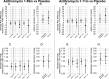
Results: Underweight subgroups had higher overall mortality compared with non-underweight subgroups. IRDs of deaths in children aged 1-11 months comparing communities receiving azithromycin to children 1-59 months of age to placebo were -6.2 deaths per 1000 person-years (95% CI -9.3 to -2.6) overall, -8.0 (95% CI -15.9 to -0.4) in the moderate to severe subgroup and -11.2 (95% CI -26.0 to -2.1) in the severe subgroup. Similar trends were noted in the azithromycin 1-11 month comparison. Malnutrition was not a statistically significant effect modifier for either comparison.
Conclusions: Although analyses suggest the potential for stronger effects in more severe underweight subgroups, we were unable to demonstrate underweight status as an effect modifier. In fact, azithromycin mass drug administration to children 1-59 months old reduced mortality in all subgroups, and, especially as the number of lives saved would be the highest by treating all subgroups, our results do not support restricting eligibility for this intervention.
Trial registration number: clinicaltrials.gov NCT04224987.
Keywords: Community child health; Mass Drug Administration; Mortality.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Sharrow D, Hug L, You D, et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob Health. 2022;10:e195–206. doi: 10.1016/S2214-109X(21)00515-5. - DOI - PMC - PubMed
