CHIKV mRNA vaccines encoding conserved structural/envelope proteins confer broad cross-lineage protection against infection
- PMID: 40148284
- PMCID: PMC11950367
- DOI: 10.1038/s41392-025-02182-2
CHIKV mRNA vaccines encoding conserved structural/envelope proteins confer broad cross-lineage protection against infection
Abstract
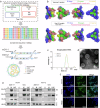
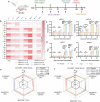
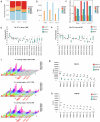
With the broad spread of the chikungunya virus (CHIKV), there is an increasing demand for more effective and broadly protective vaccines. Here, we designed CHIKV mRNA vaccines containing full-length structural proteins or part of structural proteins (envelope proteins) based on conserved sequences from 769 viral strains encompassing four lineages. The vaccine induced strong cellular and humoral immune responses in BALB/c mice and provided robust protection. Immunization of BALB/c mice with either of the two vaccines induced high levels of neutralizing antibodies against pseudoviruses from four distinct lineages, highlighting their potential for broad cross-lineage protective efficacy. Immunoglobulin repertoire analysis revealed two important BCR V-J gene combinations, IgHV1-4-IgHJ3 and IgHV1-4-IgHJ2, and lineage-specific immunity analysis revealed significant upregulation of TCRs containing V19 and V20. BCR and TCR immunodiversity may be a potential reason for the broad-spectrum protection against CHIKV afforded by the vaccine. In A129 mice, it elicited lower levels of neutralizing antibodies but prevented mouse mortality and cleared chronic infection. In the rhesus macaque model, both vaccines elicited a certain level of humoral and cellular immune responses and protected the rhesus macaques from the CHIKV challenge. In conclusion, the results from both mouse and rhesus macaque models indicate that the vaccine could be a candidate for clinical use against CHIKV.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Suhrbier, A. Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat. Rev. Rheumatol.15, 597–611 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

