Rotenone induced acute miRNA alterations in extracellular vesicles produce mitochondrial dysfunction and cell death
- PMID: 40148337
- PMCID: PMC11950519
- DOI: 10.1038/s41531-025-00917-0
Rotenone induced acute miRNA alterations in extracellular vesicles produce mitochondrial dysfunction and cell death
Abstract
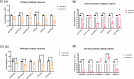
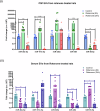
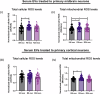
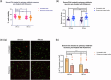
How extracellular vesicles (EVs) may contribute to mechanisms of primary intracellular pathogenesis in Parkinson's disease (PD) remains unknown. To critically advance our understanding of how EVs influence early-stage PD pathogenesis, we tested the hypothesis that rats acutely exposed to the PD neurotoxin rotenone would produce differential miRNAs in CSF/serum-derived EVs and that such modulation would be responsible for PD-relevant functional alterations in recipient neuronal cells. We discovered that acute rotenone treatment produced significant and specific serum miRNA alterations. Primary midbrain neurons treated with serum EVs from rotenone-exposed rats produced oxidative stress, mitochondrial toxicity, and cell loss in neuronal culture. These mechanisms were dependent on miR-30a-5p and miR-484. Thus, this study has elucidated that differential expression of miRNAs in circulating EVs from serum/CSF of rats is a potential early diagnostic marker for PD, and that the modulation of cellular functions and viability due to extracellular vesicles determines the pathological fate.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet397, 2284–2303 (2021). - PubMed
-
- Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson disease: a review. JAMA323, 548 (2020). - PubMed
-
- Oertel, W. & Schulz, J. B. Current and experimental treatments of Parkinson disease: a guide for neuroscientists. J. Neurochemistry139, 325–337 (2016). - PubMed
-
- Jamebozorgi, K. et al. Cellular and molecular aspects of Parkinson treatment: future therapeutic perspectives. Mol. Neurobiol.56, 4799–4811 (2019). - PubMed
Grants and funding
- R01ES03501/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01ES025750/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01 ES035019/ES/NIEHS NIH HHS/United States
- R01ES033173/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01 ES025750/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources

