Miscible chemical ordering in Ti-Cr-Mo quinary system by solid solution of Mo2Ti2AlC3 and Cr2.5Ti1.5AlC3 o-MAXs
- PMID: 40148354
- PMCID: PMC11950389
- DOI: 10.1038/s41467-025-58242-2
Miscible chemical ordering in Ti-Cr-Mo quinary system by solid solution of Mo2Ti2AlC3 and Cr2.5Ti1.5AlC3 o-MAXs
Abstract
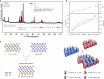
Out-of-plane ordering is promising for separately adjusting the heterodesmic chemical bonding inside the MAX phase thus tuning their properties, while constructing the out-of-plane ordered-MAX (o-MAX) is still a challenge. In this work, a strategy towards o-MAX by solid solutions of two existing o-MAXs is verified, i.e., Cr2.5Ti1.5AlC3 and Mo2Ti2AlC3, with controllable stoichiometric ratios (1:2, 1:1, and 2:1). A miscible chemical ordering is observed in three Ti-Cr-Mo quinary MAXs, which inherits the out-of-plane ordering from both parental o-MAXs. Meanwhile, through density functional theory (DFT) calculations, the electronic structure and bonding states inside the quinary o-MAXs are analyzed. Based on the calculations, anisotropic and improved mechanical properties are predicted, which agree with the experimental observed high compressive strength and tunable capacity of energy dissipation. The present work proves a promising way for synthesizing multicomponent o-MAXs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Ma, C. et al. Atomic level out-diffusion and interfacial reactions of MAX phases in contact with metals and air. J. Eur. Ceram. Soc.44, 1–22 (2024).
-
- Tan, Q. et al. Recent progress in additive manufacturing of bulk MAX phase components: A review. J. Mater. Sci. Technol.131, 30–47 (2022).
-
- Zhang, Z. et al. On the formation mechanisms and properties of MAX phases: A review. J. Eur. Ceram. Soc.41, 3851–3878 (2021).
-
- Kubitza, N. et al. Extending the chemistry of layered solids and nanosheets: chemistry and structure of MAX phases, MAB phases and MXenes. Chem. Plus Chem.88, e202300214 (2023). - PubMed
-
- Yu, H. et al. Mapping the structure and chemical composition of MAX phase ceramics for their high-temperature tribological behaviors. Carbon Energy6, e597 (2024).
Grants and funding
- 52275212/National Natural Science Foundation of China (National Science Foundation of China)
- 2024RS-CXTD-63/Natural Science Foundation of Shaanxi Province (Shaanxi Province Natural Science Foundation)
- D5000230047/Ministry of Education of the People's Republic of China (Ministry of Education of China)
- 2023T012/Northwestern Polytechnical University (NPU)
LinkOut - more resources
Full Text Sources

