Leveraging electronic medical records to evaluate a computerized decision support system for staphylococcus bacteremia
- PMID: 40148479
- PMCID: PMC11950190
- DOI: 10.1038/s41746-025-01569-3
Leveraging electronic medical records to evaluate a computerized decision support system for staphylococcus bacteremia
Abstract
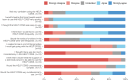
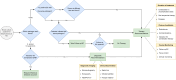
Infectious disease specialists (IDS) improve outcomes of patients with Staphylococcus bacteremia, but immediate IDS access is not always guaranteed. We investigated whether a care-integrated computerized decision support system (CDSS) can safely enhance the standard of care (SOC) for these patients. We conducted a multicenter, noninferiority, interventional stepped-wedge cluster randomized controlled trial relying on the data integration centers at five university hospitals. By this means, electronic medical records can be used for part of the trial documentation. We analyzed 5056 patients from 134 wards (Staphylococcus aureus (SAB): n = 812, coagulase-negative staphylococci (CoNS): n = 4244) and found that the CDSS was noninferior to the SOC for hospital mortality in all patients. Noninferiority regarding the 90-day mortality/relapse in SAB patients was not observed and there was no evidence for differences in vancomycin usage among CoNS patients. Despite low reported usage, physicians rated the CDSS's usability favorably. Trial registration: drks.de; Identifier: DRKS00014320; Registration Date: 2019-05-06.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: O.W. has received research grants for clinical studies, speaker fees, honoraria and travel expenses from Amgen, Alexion, Astellas, Astra Zeneca, Basilea, Biotest, Bristol-Myers Squibb, Correvio, Chiesi, Gilead, GSK; Hexal, Janssen, Dr. F. Köhler Chemie, MSD, Novartis, Roche, Pfizer, Sanofi, Takeda, TEVA, Tillotts Pharma and UCB. OW is supported by an unrestricted grant from the Rudolf-Ackermann-Stiftung (Stiftung für Klinische Infektiologie). MPl has received speaker fees and honoraria from MSD, Pfizer, GSK, Gilad, Thermo Fisher, Infectopharm, Roche and BioNtech and has received a Pfizer grant for a study on CAP. SH has received speaker fees from Pfizer, MSD, Infectopharm, Philips, Advanz, Beckman Coulter, Shionogi, and Tillots. G.M. has received speaker fees and honoraria from März AG, BBraun Melsungen and 4TEEN4. The remaining authors declare no competing interests.
Figures




References
-
- Diekema, D. J. et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific Region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis.32, S114–S132 (2001). - PubMed
-
- Schmitt, S. et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin. Infect. Dis.58, 22–28 (2014). - PubMed
-
- Vogel, M. et al. Infectious disease consultation for Staphylococcus aureus bacteremia—a systematic review and meta-analysis. J. Infect.72, 19–28 (2016). - PubMed
-
- Benfield, T. et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin. Microbiol. Infect.13, 257–263 (2007). - PubMed
-
- López-Cortés, L. E. et al. Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin. Infect. Dis.57, 1225–1233 (2013). - PubMed
Grants and funding
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803N/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803P/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803P/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803P/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803N/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803N/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803N/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803N/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803P/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803N/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803N/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803P/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803P/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01ZZ1803C/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources

