Factor H-related 2 levels dictate FHR dimer composition
- PMID: 40148491
- PMCID: PMC11950314
- DOI: 10.1038/s41598-025-94064-4
Factor H-related 2 levels dictate FHR dimer composition
Abstract
Factor H-related (FHR) protein 1 and 2 form dimers resulting in FHR-1 and -2 homodimers, and FHR-1/2 heterodimers. Dimerization is hypothesized to further increase their antagonistic function with complement regulator factor H (FH). So far, only FHR-1 homodimers and FHR-1/2 heterodimers could be quantified in a direct way. With the reported genetic associations between CFHR2 and complement-related diseases such as age related macular degeneration and C3-glomerulopathy, direct assessment of FHR-2/2 levels determining the dimer distribution of FHR-1 and -2 is needed to further elucidate their role within complement regulation. Therefore, novel in-house generated FHR-2 antibodies were used to develop a specific ELISA to enable direct quantification of FHR-2 homodimers. Allowing for the first time the accurate measurement of all FHR-1 and -2 containing dimers in a large cohort of healthy donors. By using native FHR-1 and -2 or deficient plasma, we determined the stability, kinetics and distribution of FHR-1 and -2 dimers. Additionally, we show how genetic variants influence dimer levels. Our results confirm a rapid, dynamic, dimer formation in plasma and show FHR-1/2 dimerization rearches a distribution equilibrium that is limited by the relative low levels of FHR-2 in relation to its dimerization partner FHR-1.
Keywords: Complement system; Dimerization; FHR-1; FHR-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: MCB, TWK and RBP are co-inventors of patents and patents applications describing potentiating anti-FH antibodies and uses thereof. All other authors declare no conflict of interest. Ethical approval: After consultation with the ethical board of Sanquin Research, Amsterdam, The Netherlands, a system was established for obtaining blood samples for scientific research (no approval number available). This volunteer system is organized according to Dutch regulations and according to the Declaration of Helsinki. This volunteer system certifies, among others, that: Blood samples used for scientific studies by researchers of the Sanquin Research department were drawn from healthy, anonymized volunteers with written informed consent; No personal characteristics of the volunteers are registered; The volunteers nor those taking the samples know for what project specific samples are used; Allowed annual sample volume and frequency of donation were established after consultation with Sanquin Medical Secretary. Standard operating procedures are available upon request.
Figures
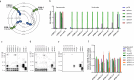
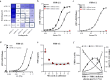
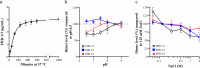
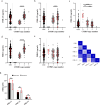
References
-
- Zipfel, P. F. & Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol.9(10), 729–740. 10.1038/nri2620 (2009). - PubMed
-
- Józsi, M., Schneider, A. E., Kárpáti, É. & Sándor, N. Complement factor H family proteins in their non-canonical role as modulators of cellular functions. Semin. Cell Dev. Biol.10.1016/j.semcdb.2017.12.018 (2019). - PubMed
-
- Krushkal, J., Bat, O. & Gigli, I. Evolutionary relationships among proteins encoded by the regulator of complement activation gene cluster. Mol. Biol. Evol.17(11), 1718–1730. 10.1093/OXFORDJOURNALS.MOLBEV.A026270 (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

