Long-term immune responses to SARS-CoV-2 Omicron BA.4/5 mRNA booster in people living with HIV
- PMID: 40148493
- PMCID: PMC11950219
- DOI: 10.1038/s43856-025-00799-6
Long-term immune responses to SARS-CoV-2 Omicron BA.4/5 mRNA booster in people living with HIV
Abstract
Background: Variant-adapted vaccines are recommended in vulnerable populations to address the waning immunity and the emergence of immune-escaping SARS-CoV-2 variants, yet data about immune responses to such vaccines in people living with HIV (PLWH) are limited. We therefore aimed to assess long-term immune responses to an original-BA.4/5 mRNA booster in this population.
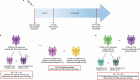
Methods: In this prospective longitudinal study, PLWH receiving either an original-BA.4/5 bivalent booster or an original monovalent booster and HIV-negative healthcare workers (HCWs) receiving a bivalent booster were enrolled and sampled before (T0), 1 month (T1), and 4-9 months (T2) after the vaccine administration. SARS-CoV-2-specific T and B cells, RBD-binding antibodies, and RBD-blocking antibodies against both wild type (WT) and omicron BA.4/5 virus were determined.
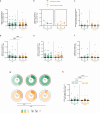
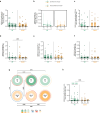
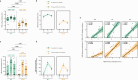
Results: The bivalent booster is able to transiently increase both humoral and polyfunctional T cell responses in PLWH, with humoral responses comparable to those observed in HCWs. While T cell responses are cross-reactive against viral variants and stable over time, humoral immunity is imprinted to the ancestral virus and wanes quickly. Furthermore, whilst previous SARS-CoV-2 infection does not affect the trajectory of vaccine-elicited immune responses, markers of HIV-related T cell dysfunction are associated with lower antibody peak responses and higher antibody waning. Lastly, the bivalent booster was superior to the monovalent one in inducing BA.4/5-reactive RBD-blocking antibodies.
Conclusions: The original-BA.4/5 bivalent booster is highly immunogenic in PLWH and superior to the monovalent one in inducing humoral responses against the BA.4/5 virus, although HIV-related T cell dysfunction markers are associated with blunted and less durable antibody immunity.
Plain language summary
SARS-CoV-2 vaccines adapted to recently circulating variants are recommended in vulnerable populations, such as people living with HIV (PLWH). In this study, we studied immune responses to a newly designed mRNA vaccine in this cohort. We showed that the vaccine could stimulate antibodies, small proteins that the body produces against the SAR-CoV-2 original and the mutant BA.4/5 variants to fight the virus. This new design produced improved antibody responses against SARS-CoV-2 mutant variants compared with older designs, but PLWH with a compromised immune system have a short-lived protection against the evolving virus.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

